Immunoassay method and kit for assaying mycobacterium tuberculosis from biological samples
A technology of Mycobacterium tuberculosis and an immune detection method, applied in the field of medical immunodiagnostics, can solve the problems of high complexity of molecular detection, inability to obtain higher detection rate specificity, inability to obtain bacteriological evidence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
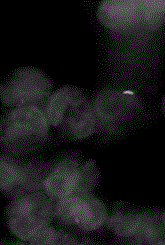
[0048] Detection and Identification of Mycobacterium tuberculosis in Example 1 Plasma
[0049] (1) Collect 5-10ml of the subject's blood (anticoagulation, EDTA anticoagulation can help to detect Mycobacterium tuberculosis and then do molecular detection and drug resistance test);
[0050] (2) Centrifuge at 500-1500 rpm for 5-10 minutes (it can also allow blood cells to sink naturally and then separate plasma and blood cells);
[0051] (3) Based on microporous membrane (the material of microporous membrane can be any suitable material, such as polycarbonate membrane, cellulose ester membrane, polyamide membrane, polysulfone membrane, fluorine-containing material membrane, polyester membrane Membranes, polyolefin membranes, inorganic material membranes, etc., preferably polycarbonate membranes.) microfluidic filtration device to filter and enrich Mycobacterium tuberculosis;
[0052] (4) Plasma 2-10ml, take 2ml as an example to illustrate the method and process;
[0053] (5) Ad...
Embodiment 2
[0062] Example 2 Detection and identification of mycobacterium tuberculosis in cerebrospinal fluid
[0063] (1) 0.5-2ml of freshly collected cerebrospinal fluid, to avoid cell damage, decomposition of glucose and other substances, bacterial dissolution, etc. due to the specimen being placed for too long, and the specimen should try to avoid coagulation and mixing into the blood;
[0064] (2) Membrane-based microfluidic filtration device to filter and enrich Mycobacterium tuberculosis;
[0065] (3) Cerebrospinal fluid 0.5-2ml, take 0.5ml as an example to illustrate the method and process;
[0066] (4) Add 0.5ml of cerebrospinal fluid to 3.5ml of normal saline or PBS, and mix well;
[0067] (5) Add 8ml of 6% sodium hypochlorite, mix well, and let stand for 5-20 minutes;
[0068] (6) Add 48ml of 95 ethanol (containing 1% TritonX-100), mix well, and let stand for 5-15 minutes;
[0069] (7) Add the above-mentioned liquid into a microfluidic device or filter and enrich Mycobacter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com