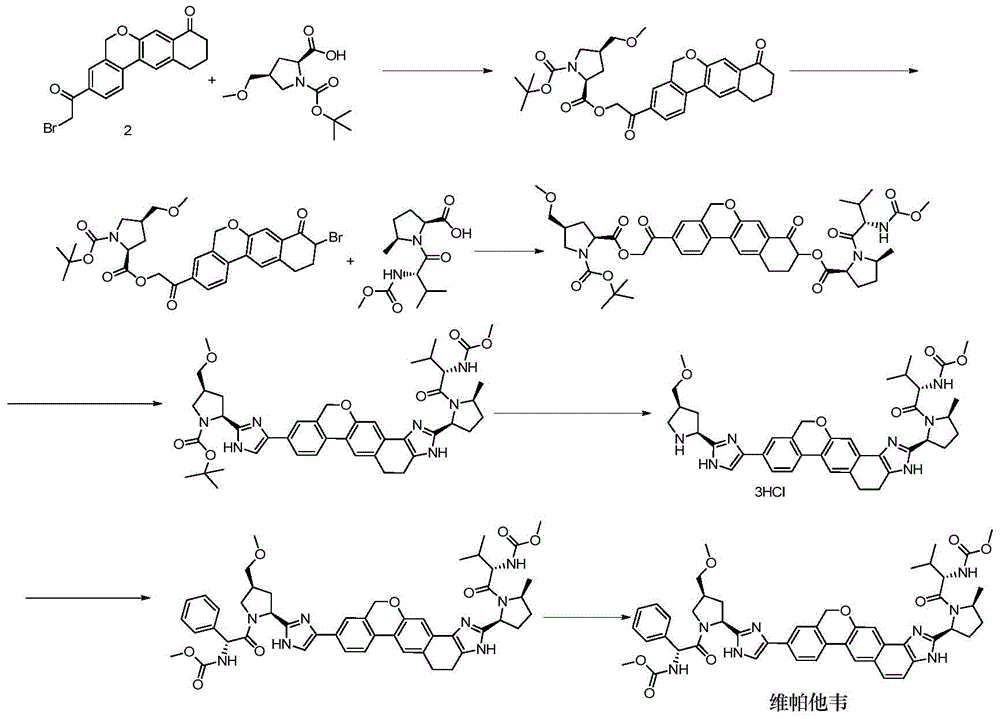
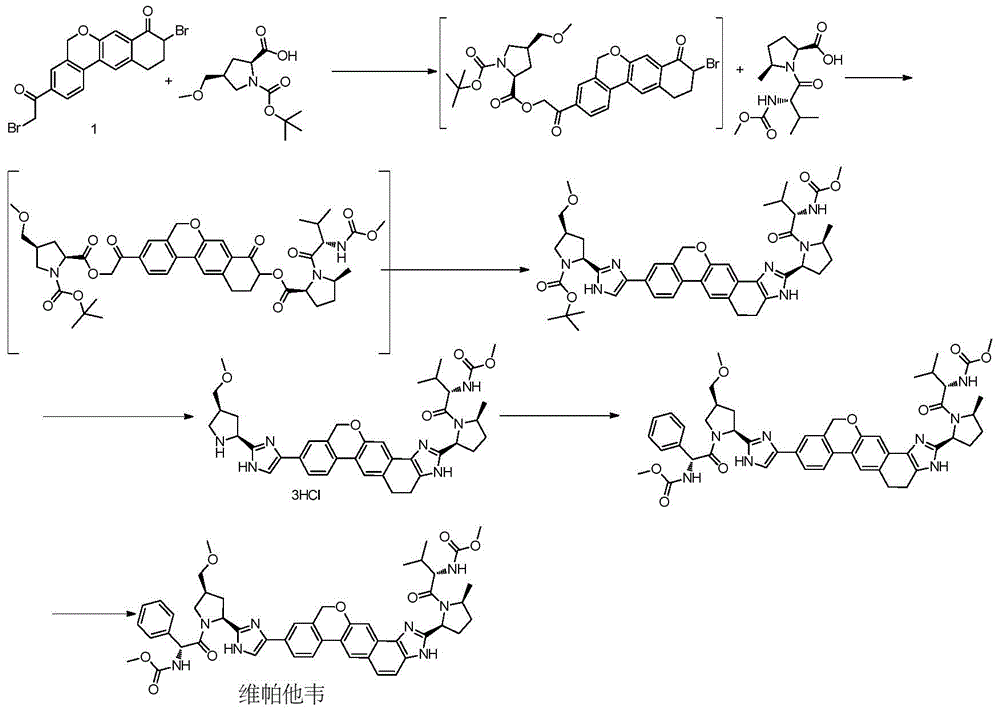
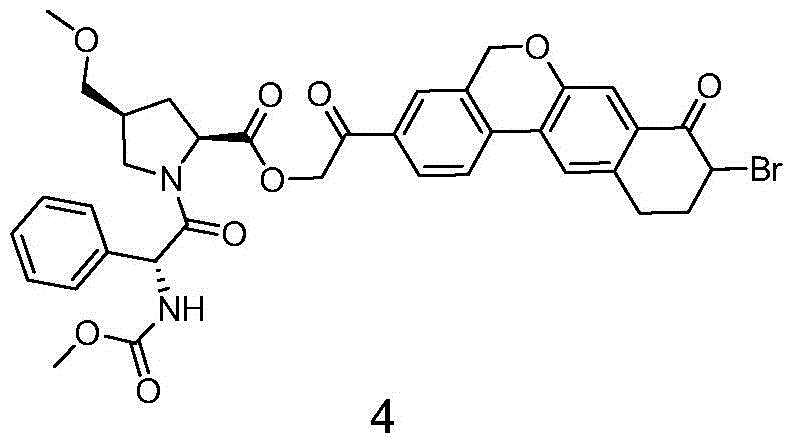
Novel synthesis method of hepatitis drug velpatasvir
A synthesis method and velpatasvir technology are applied in the field of new method synthesis of a new hepatitis C virus drug velpatasvir and a series of intermediates thereof, which can solve the problems of increasing the pressure of subsequent reaction purification, high cost and low efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]
[0046] Add compound 31 (45.01g, 100mmol), compound 3 (35.04, 100mmol), N,N-dimethylformamide (225mL) into the three-necked flask, stir well, add potassium carbonate (27.64g, 200mmol), and react at room temperature 6 -8 hours. Add water (450mL) and ethyl acetate (450mL) after the reaction, separate the layers, extract the aqueous layer once with ethyl acetate (225mL), combine the organic phases and wash with water (225mL) twice, dry over anhydrous sodium sulfate, filter, and concentrate Most of the ethyl acetate was removed, petroleum ether (450 mL) was added to make a slurry, filtered, and vacuum-dried to obtain intermediate 4 (56.13 g, yield 78%). MS(ESI)m / z719[M+H] + , 1 HNMR(CDCl3,400MHz)7.95-7.80(m,2H),7.75-7.65(m,2H),7.50-7.40(m,2H),7.40-7.25(m,4H),6.20-5.90(m,1H) ,5.55-5.45(m,2H),5.34-5.22(m,1H),5.16(s,2H),4.77-4.50(m,2H),3.85-3.77(m,1H),3.65-3.61(m, 3H), 3.40-3.15(m, 6H), 2.98-2.88(m, 2H), 2.65-2.45(m, 3H), 1.98-1.83(m, 2H).
Embodiment 2
[0048]
[0049]Add compound 4 (35.98g, 50mmol) in the three-necked flask, compound 5 (15.03g, 52.5mmol), N,N-dimethylformamide (180mL), stir well and add cesium carbonate (32.58g, 100mmol), heat React at 50°C for 4-6 hours, cool to room temperature after the reaction, add saturated ammonium chloride (360mL), ethyl acetate (360mL), separate the layers, extract the water layer with ethyl acetate (180mL) once more, combine the organic phases and wash with water (180mL) twice, dried over anhydrous sodium sulfate, filtered, concentrated to remove most of the ethyl acetate, added petroleum ether (360mL) for slurry, filtered, and vacuum-dried to give intermediate 6 (34.69g, yield 75%). MS(ESI)m / z925[M+H] + , 1 HNMR(CDCl3,400MHz)8.00-7.60(m,4H),7.58(s,1H),7.47-7.25(m,5H),6.25-5.95(m,1H),5.63-5.45(m,3H),5.30 -5.22(m,2H),5.16(s,2H),4.75-4.55(m,2H),4.35-4.15(m,1H),3.86-3.77(m,1H),3.72-3.58(m,6H) ,3.40-3.05(m,7H),2.91(m,1H),2.62-1.80(m,10H),1.45-1.35(m,3H),1.05-0.80(m,6H).
Embodiment 3
[0051]
[0052] Add compound 4 (35.98g, 50mmol) in the three-necked flask, compound 9 (12.04g, 52.5mmol), N,N-dimethylformamide (180mL), stir well and add cesium carbonate (32.58g, 100mmol), heat React at 50°C for 4-6 hours, cool to room temperature after the reaction, add saturated ammonium chloride (360mL), ethyl acetate (360mL), separate the layers, extract the water layer with ethyl acetate (180mL) once more, combine the organic phases and wash with water (180mL) twice, dried over anhydrous sodium sulfate, filtered, concentrated to remove most of the ethyl acetate, added petroleum ether (360mL) for slurry, filtered, and vacuum-dried to give intermediate 10 (31.96g, yield 72%). MS(ESI)m / z868[M+H] + , 1 HNMR(CDCl3,400MHz)δ7.98-7.80(m,2H),7.98-7.59(m,3H),7.44(d,J=7.6Hz,2H),7.37-7.27(m,3H),6.24-5.90 (m,1H),5.65-5.45(m,3H),5.28-5.21(m,1H),5.17(s,2H),4.76-4.63(m,1H),3.90-3.70(m,1H),4.52 -4.30(m,1H),4.11-3.90(m,1H),3.85-3.77(m,1H),3.70-3.60(m,3H),3.40-3.10(m,7H),2.95-2.87(m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com