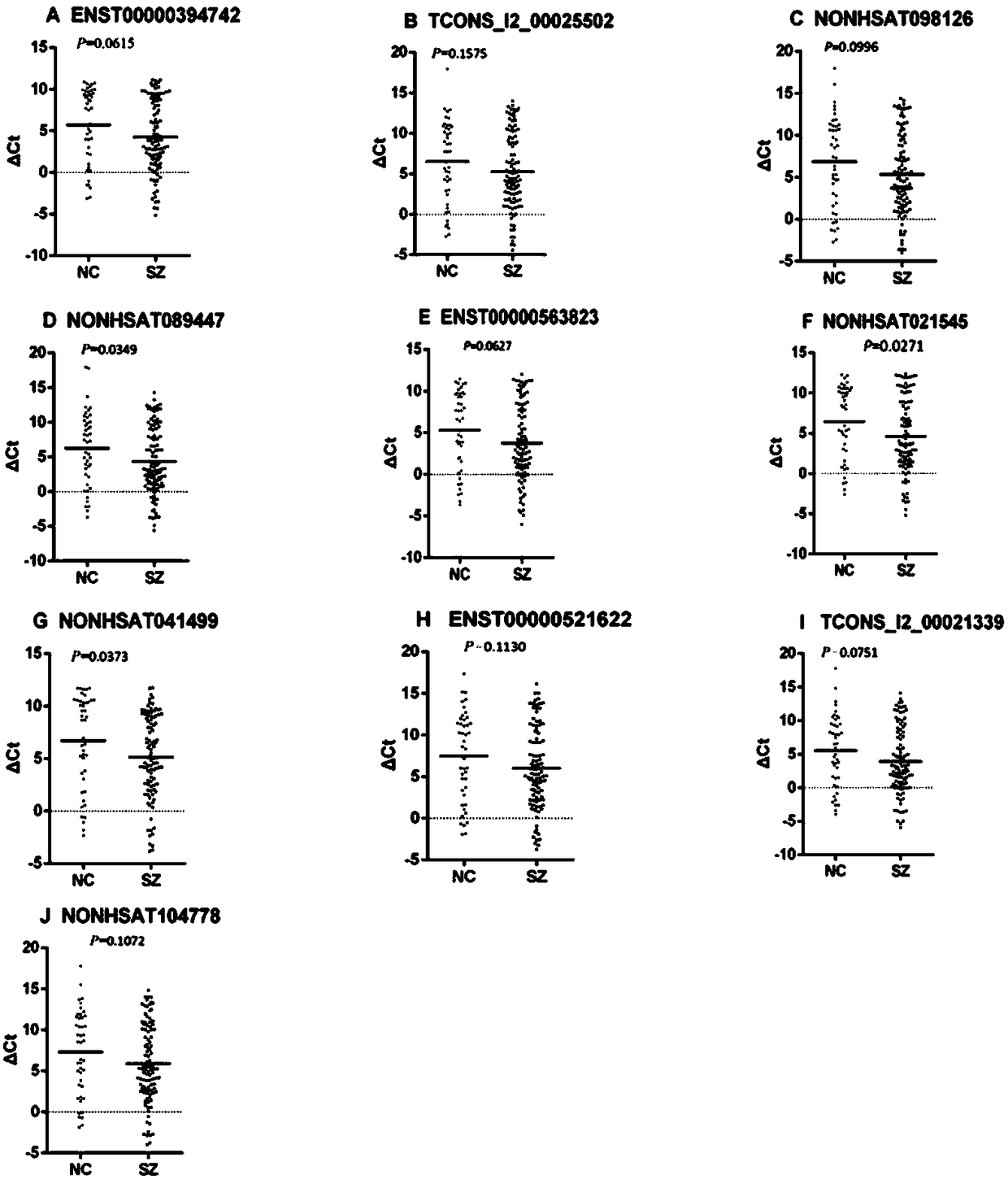
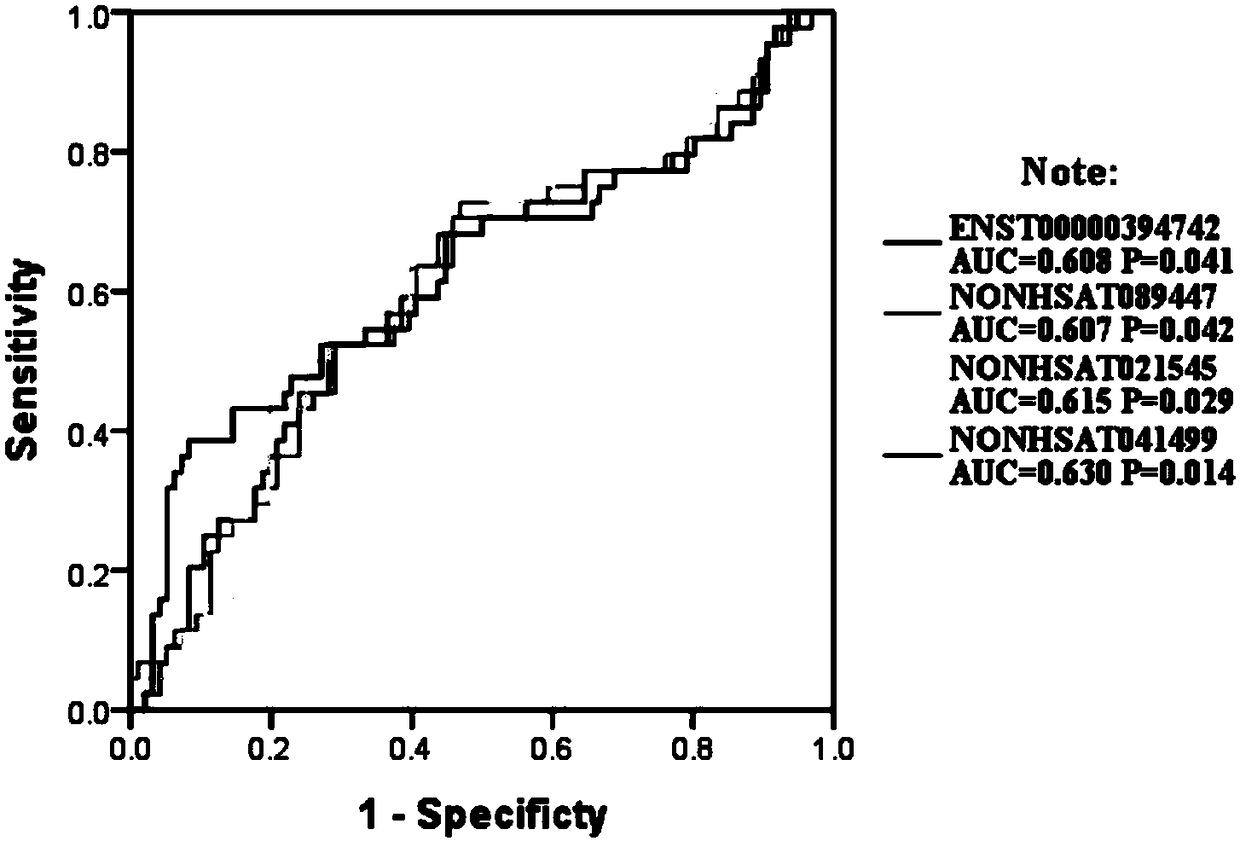
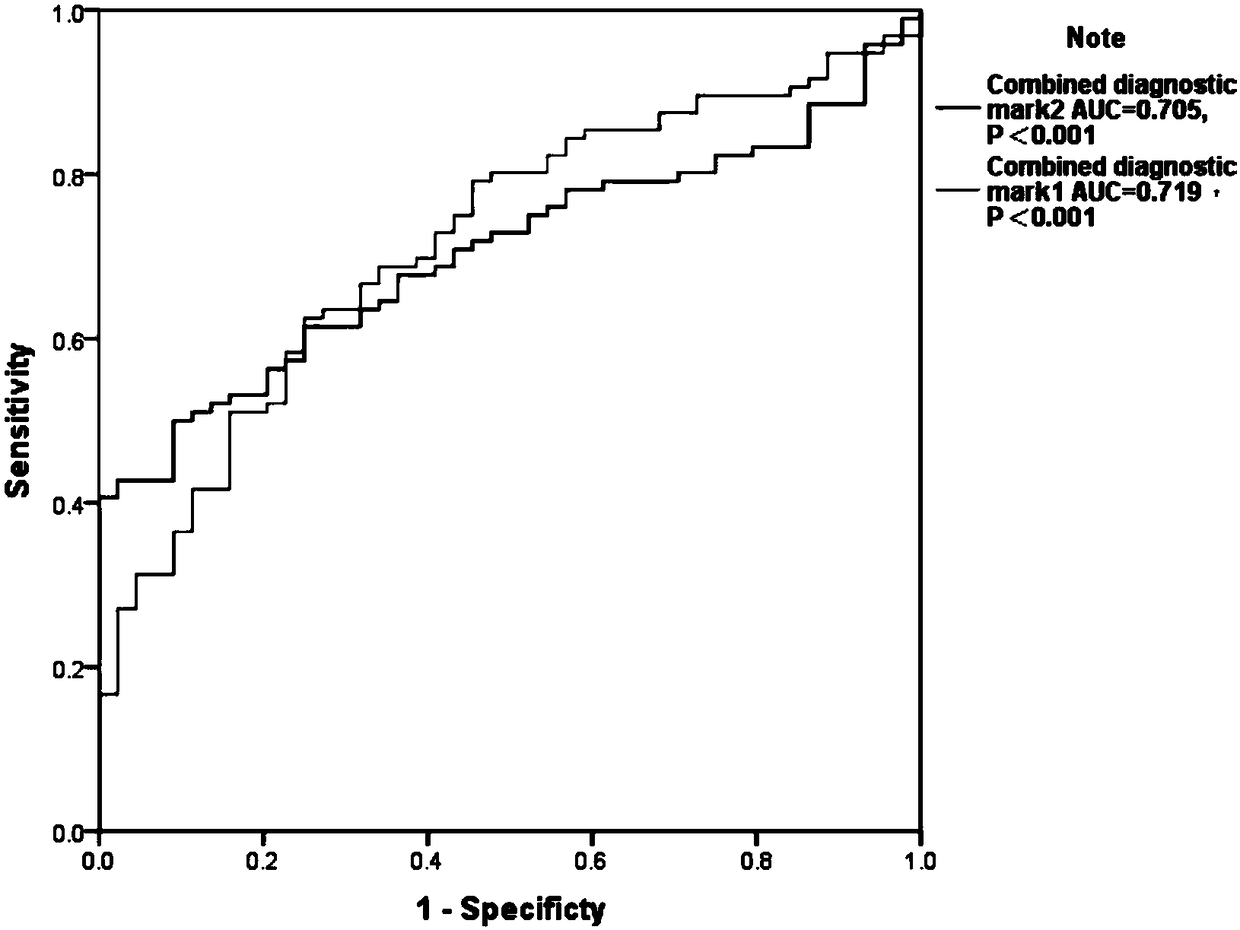
A lncrna marker and kit for schizophrenia diagnosis
A technology for schizophrenia and diagnostic kits, applied in DNA/RNA fragments, recombinant DNA technology, biochemical equipment and methods, etc., can solve problems such as reducing the stability of target mRNA, achieve high diagnostic reference value, and improve diagnostic accuracy efficiency, simplifying the diagnostic process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] 1. Research object
[0031] This study was approved by the Medical Ethics Committee of the 102nd Hospital of the Second Military Medical University Clinical Hospital of the Chinese People's Liberation Army, and all subjects or their family members (guardians) signed the informed consent.
[0032]1. Case group: From August 2012 to June 2014, patients were continuously admitted to the outpatient department and psychiatric ward of the 102nd Hospital of the People's Liberation Army. Inclusion criteria: ①Meet the diagnostic criteria for schizophrenia in the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV); ②First-episode patients or did not take antipsychotic drugs 3 months before enrollment; ③Age between 15 and 80 years old. Exclusion criteria: ① suffering from other mental diseases; ② suffering from physical or nervous system diseases such as traumatic brain injury; ③ having a history of alcoholism or drug abuse; ④ having a history of blood...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com