A meso-position long-chain alkoxyphenyl tetraphenanthrene ring conjugated porphyrin derivative and its preparation method
A technology of alkoxyphenyl tetraphenanthrene ring and long-chain alkoxy aromatic aldehyde is applied in the field of preparation of long-chain alkoxyphenyl tetraphenanthrene ring conjugated porphyrin derivatives at meso position, and can solve the problem of porphyrin There are many synthetic steps, few synthetic studies, and difficult separation, etc., to achieve the effects of high yield, simple and economical operation, and good reaction selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
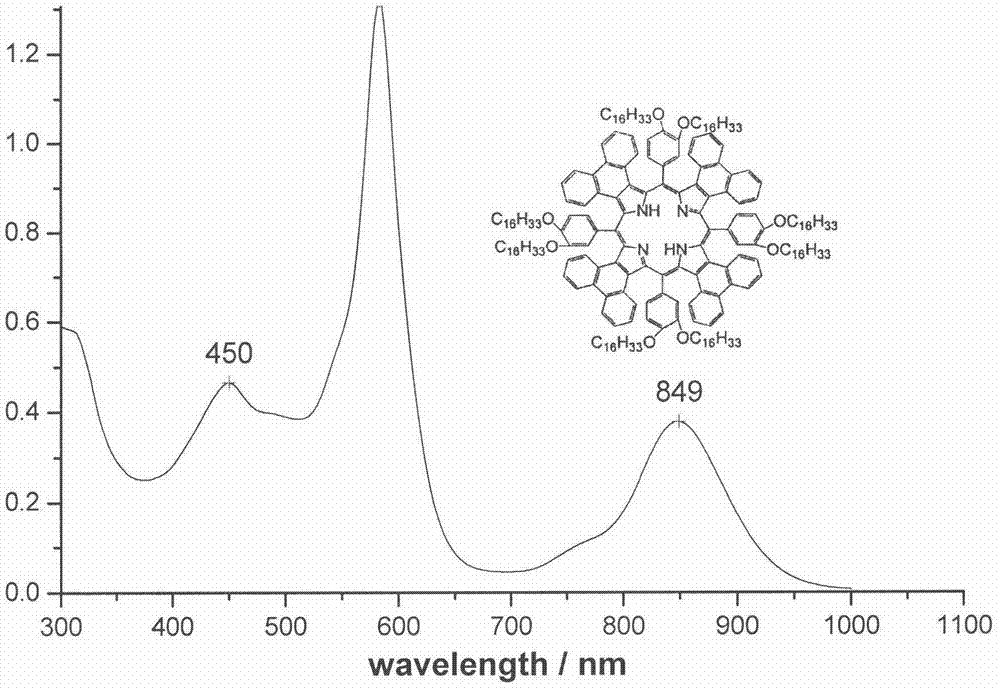
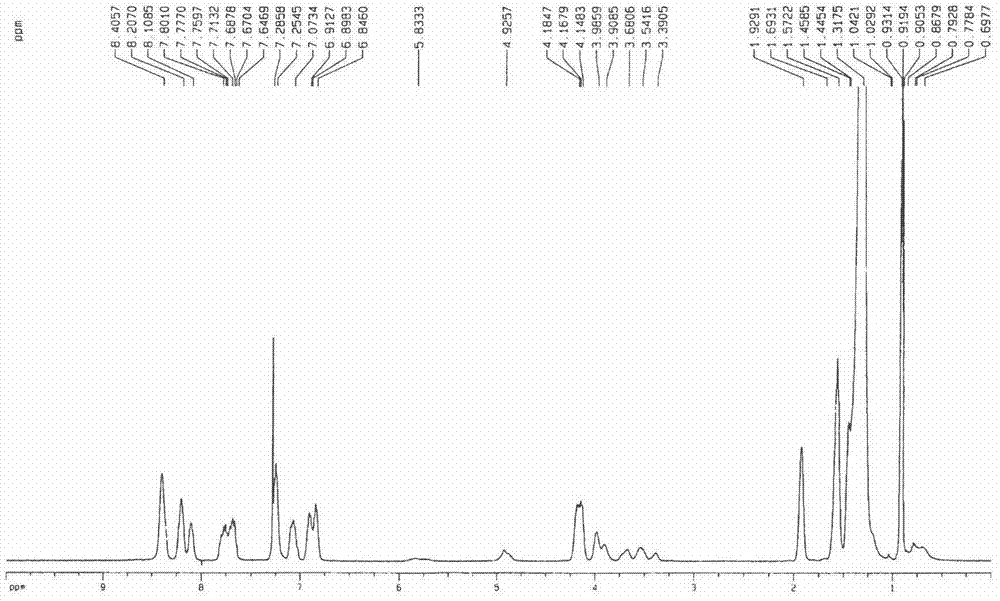
[0035] Synthesis of compound 1a: 3,4-bis(hexadecyloxy) (1.173g, 2mmol) benzaldehyde and phenanthrene ring conjugated pyrrole (0.572g, 2mmol) were added in a 50mL single-necked bottle, and propionic acid ( 15mL) for dissolution. Under the protection of argon and dark conditions, the reaction was heated to reflux for 2 h. Cool to room temperature, add an appropriate amount of methanol, filter, the residue is separated by silica gel column chromatography, the eluent is dichloromethane-petroleum ether, chloroform, and then recrystallized by methanol and chloroform to obtain a dark red product. Yield: 28.0%. mp>250℃.UV-vis(CHCl 3 )λmax nm(ε×10 -5 )583(1.319), 849(0.378)( figure 1 ); 1 H NMR (CDCl 3 )δ8.53(s, 8H), 8.34(m, 4H), 8.21-8.24(m, 4H), 7.80-7.93(m, 8H), 7.39(m, 8H), 7.20-7.23(m, 4H) , 6.98-7.04(m, 8H), 4.28-4.31(m, 8H), 4.05-4.12(m, 8H), 1.70(m, 16H), 1.45(s, 208H), 1.03-1.09(t, 24H) , 0.73(b, 2H)( figure 2 ); MALDI-TOF MASS cacd for C 220 h 310 N 4 o 8 3138....
Embodiment 2
[0037] Synthesis of compound 1b: 3,4-bis(hexadecyloxy) (1.173g, 2mmol) benzaldehyde and phenanthrene ring conjugated pyrrole (0.572g, 2mmol) were added in a 50mL single-necked bottle, and propionic acid ( 15mL) for dissolution. Under the protection of argon and dark conditions, the reaction was heated to reflux for 2 h. Cool to room temperature, add an appropriate amount of methanol, filter, the residue is separated by silica gel column chromatography, the eluent is dichloromethane-petroleum ether, chloroform, and then recrystallized by methanol and chloroform to obtain a dark red product. Yield: 25.3%. mp 220℃.UV-vis(CHCl 3 )λmax nm(ε×10 -5 )578(0.869), 809(0.161)( Figure 4 ); 1 H NMR (CDCl 3 )δ8.36-8.40 (m, 8H), 7.80-7.83 (m, 4H), 7.72-7.76 (m, 12H), 7.26-7.30 (m, 8H), 6.88-6.95 (m, 8H), 6.60- 6.68(m, 4H), 3.92-4.01(m, 8H), 3.67-3.68(m, 4H), 3.57-3.59(m, 4H), 1.58-1.59(m, 16H), 1.30-1.32(s, 210H) ), 0.90(t, 24H)( Figure 5 ); MALDI-TOF MASS cacd for C 220 h 310 N...
Embodiment 3
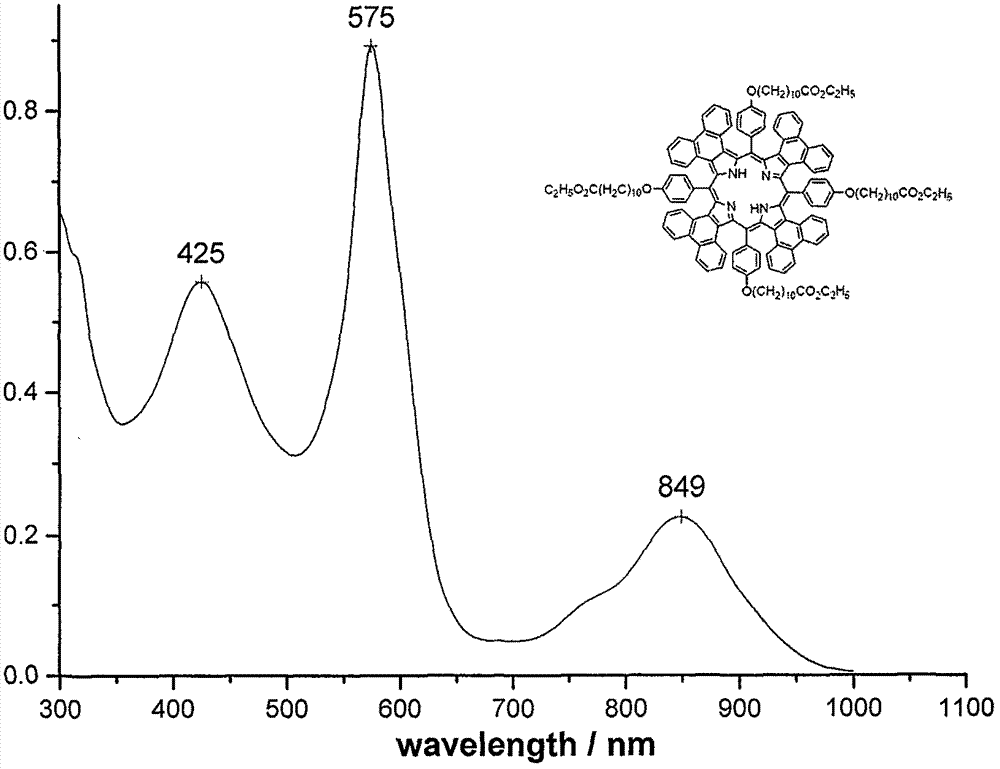
[0039] Synthesis of Compound 1c: The preparation method is the same as in Example 1, and the yield: 321.9mg, 30.2%. mp>250℃; UV-vis(CHCl 3 )λmax nm(ε×10 -5 )575(0.892), 849(0.226)( Figure 7 ); 1 H NMR (CDCl 3, 500M) 58.8-8.58(m, 8H), 8.35-8.40(m, 8H), 7.66-7.73(m, 8H), 7.23(m, 8H), 7.07-7.09(m, 8H), 6.84-6.86( m, 8H), 4.13-4.17(m, 16H), 2.28-2.35(m, 8H), 1.86-1.88(t, 8H), 1.66-1.69(m, 8H), 1.54(m, 8H), 1.38( s, 40H), 1.28(t, 12H), 0.88(b, 2H) ( Figure 8 ); MALDI-TOF MASS cacd for C 144 h 150 N 4 o 12 2128.75, found: 2131.23 (M+H + )( Figure 9 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com