Natural mineral-based catalyst and gasification method using the same
A technology of natural minerals and catalysts, applied in the field of fluidized bed gasification, to achieve the effect of easy separation and recovery, and improved gasification activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
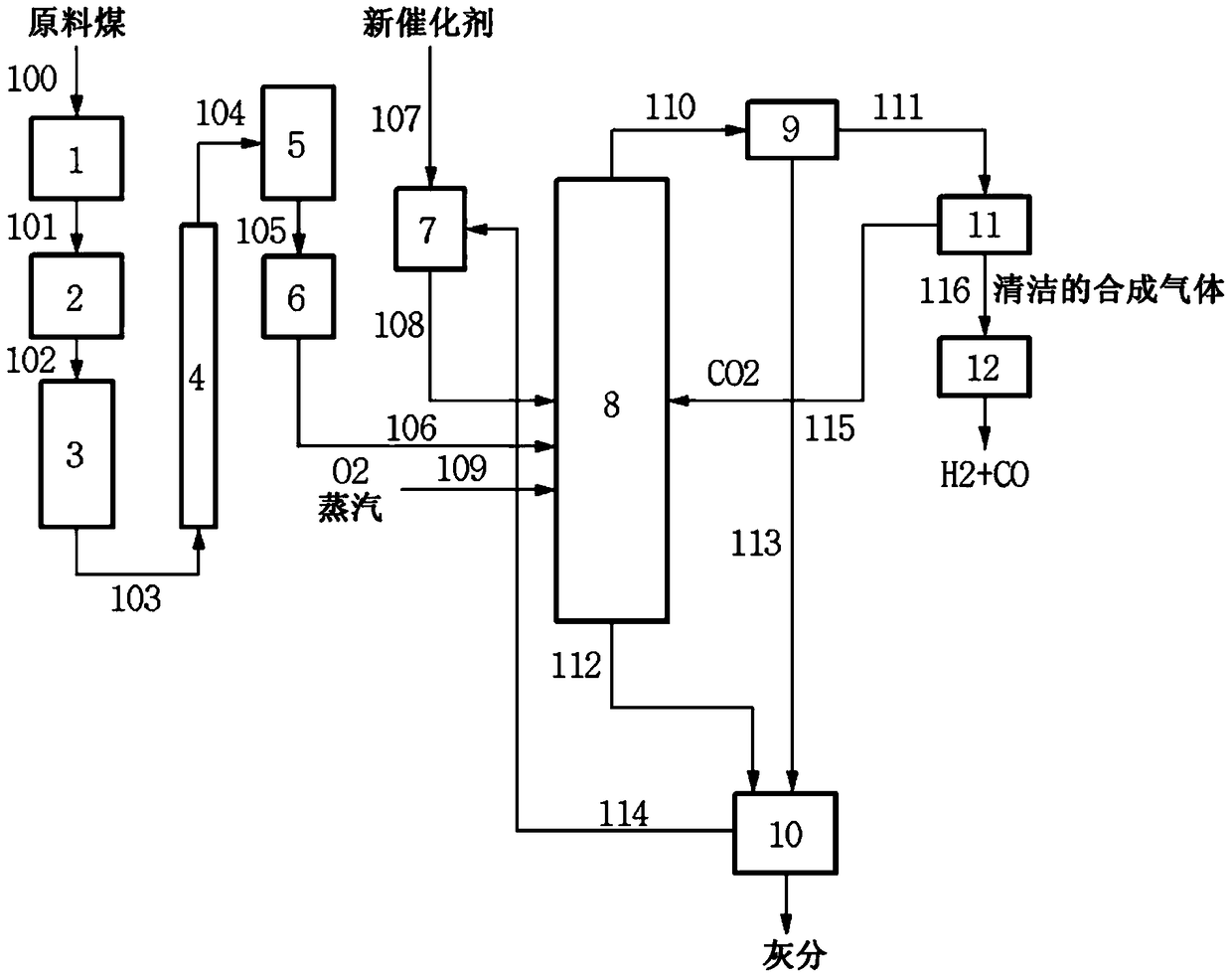
Image
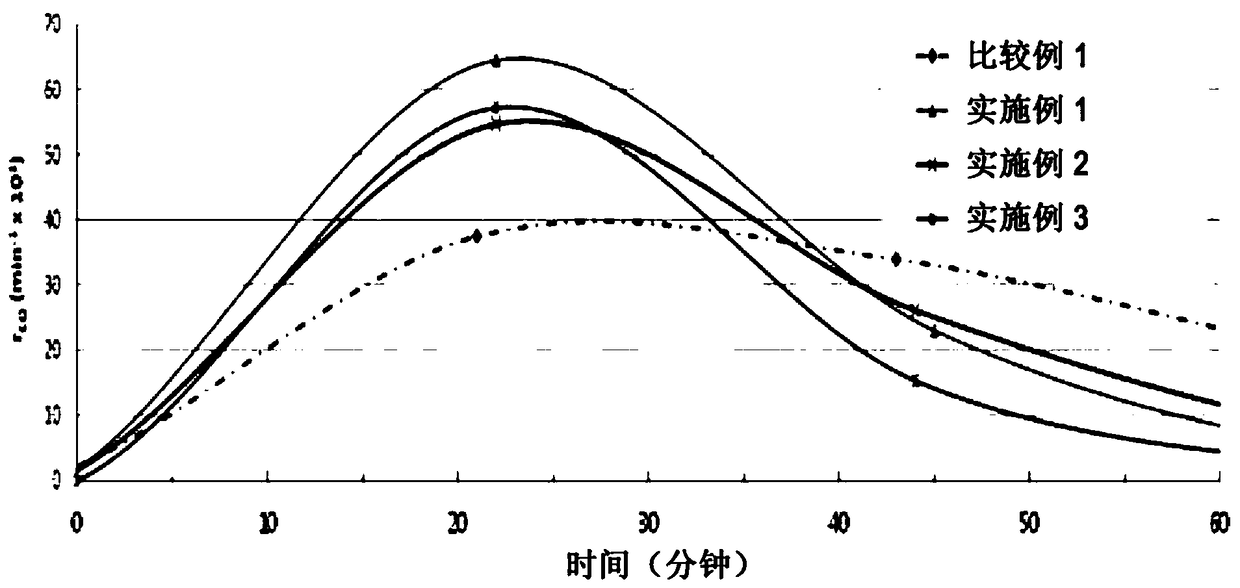
Examples
Embodiment 1
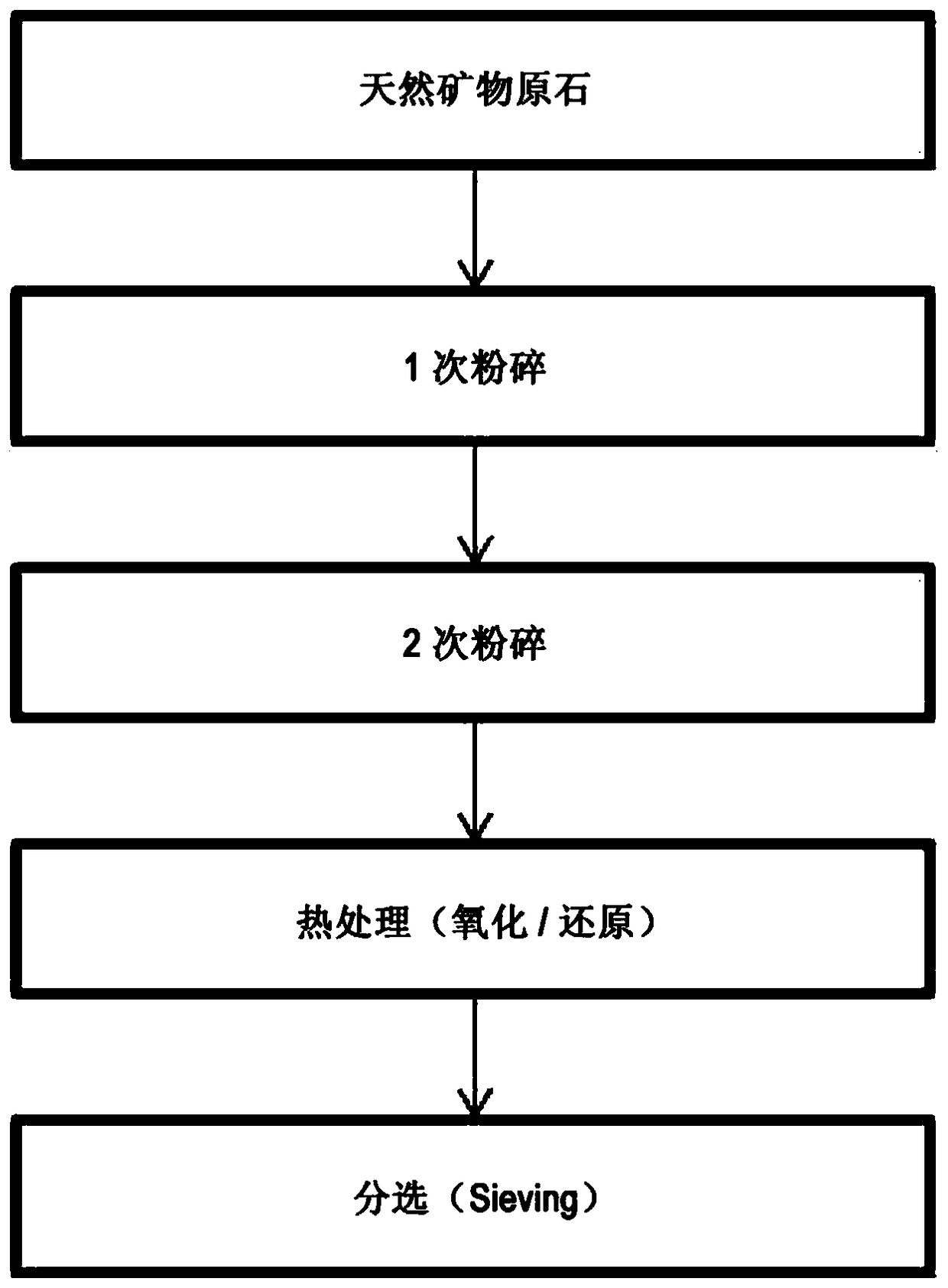
[0131] Manufacture of catalyst using natural zeolite (1)
[0132] As an aqueous Fe precursor solution, FeCl 2 4H 2 Fe of O (Sanden Pure Chemical Industries, Ltd. Iron (II) chloride tetrahydrate, 99%), and as the Mg precursor aqueous solution, prepared MgCl 2 ·6H 2 Mg of O (Magnesium chloride hexahydrate from Sanden Junyaku Co., Ltd., 98%).
[0133] Thereafter, as described above, 100 g of natural zeolite particles heat-treated at 350° C. were put into a 1-liter beaker, and then 125 g of the Fe precursor aqueous solution and 94 g of the Mg precursor aqueous solution were added thereto. The mixture was stirred at 75°C for 6 hours, filtered, and the filtrate was dried at 105°C, and then calcined in an air atmosphere at a temperature of 900°C to produce a gasification catalyst.
[0134] The composition of the catalyst was analyzed by ICP-OES (720ICP-OES from Agilent Technologies), and it was confirmed that it contained 20.5% by weight of MgO and 27.0% by weight of Fe 2 o 3...
Embodiment 2
[0136] Production of catalysts using dolomite
[0137] As an aqueous Fe precursor solution, FeCl 2 4H 2 Fe of O (Sanden Pure Chemical Industries, Ltd. Iron (II) chloride tetrahydrate, 99%), and KCl K (Sanden Pure Chemical Industries, Ltd. Potassium chloride, 99.5%).
[0138] Thereafter, 100 g of the calcined dolomite particles were put into a 1 L beaker, and then 125 g of the Fe precursor aqueous solution and 74 g of the K precursor aqueous solution were added thereto. The above-mentioned mixture was stirred at 75°C for 6 hours, filtered, and the obtained solid was dried at 105°C, and then calcined in an air atmosphere at a temperature of 950°C to produce a catalyst.
[0139] The composition of the catalyst was analyzed by ICP-OES (720ICP-OES from Agilent Technologies), and it was confirmed that it contained 24.0% by weight of MgO and 27.5% by weight of Fe 2 o 3 and 10.5% by weight of K 2 O.
Embodiment 3
[0141] Production of catalysts using eluate of natural zeolite and olivine
[0142] 100 g of the above-produced olivine particles were put into a 1 L beaker, 400 ml of 5N hydrochloric acid was added thereto, stirred at 90° C. for 3 hours, and the acid-treated mixture was filtered to separate and recover the eluate.
[0143] The contents of elements (ions) extracted from the recovered eluate sample (5 ml) are shown in Table 7 below.
[0144] 【Table 7】
[0145] element (ion)
Mg
Ca
Fe
Al
Si
Content (wt%)
0.60
0.01
0.21
0.03
0.51
[0146] 100 g of the natural zeolite particles produced above were put into a 1 L beaker, and then 200 ml of the eluate was added. Thereafter, the mixture was stirred at 75° C. for 6 hours, filtered, and the obtained solid matter was dried at 105° C., and then calcined in an air atmosphere at a temperature of 900° C. to produce a catalyst.
[0147] The composition of the catalyst was analyzed by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com