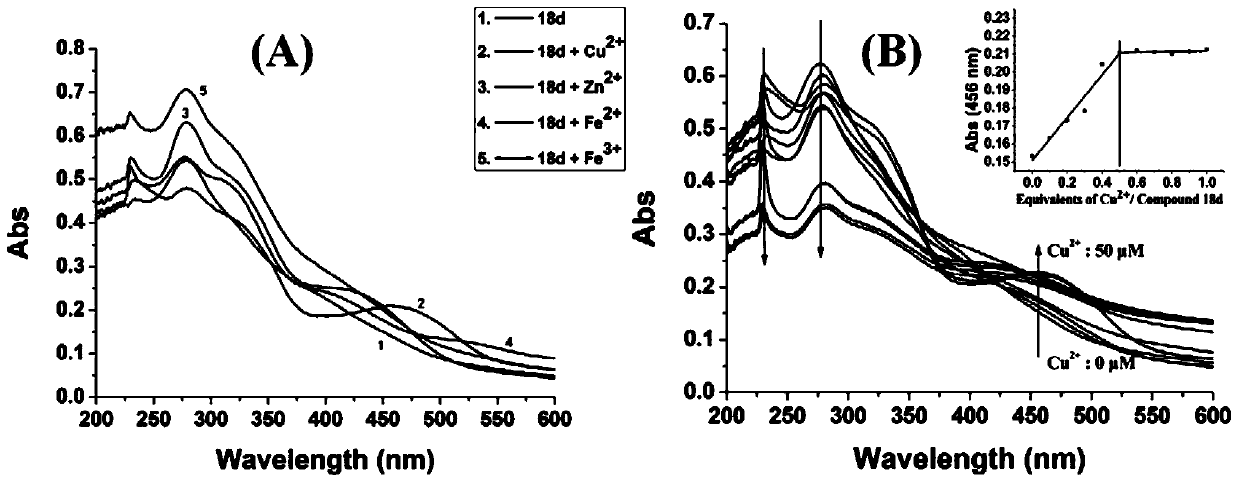
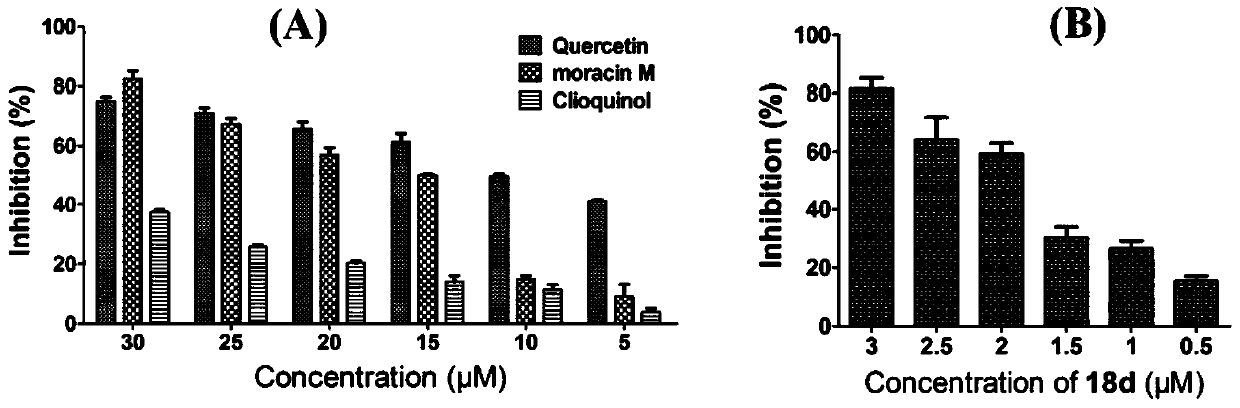
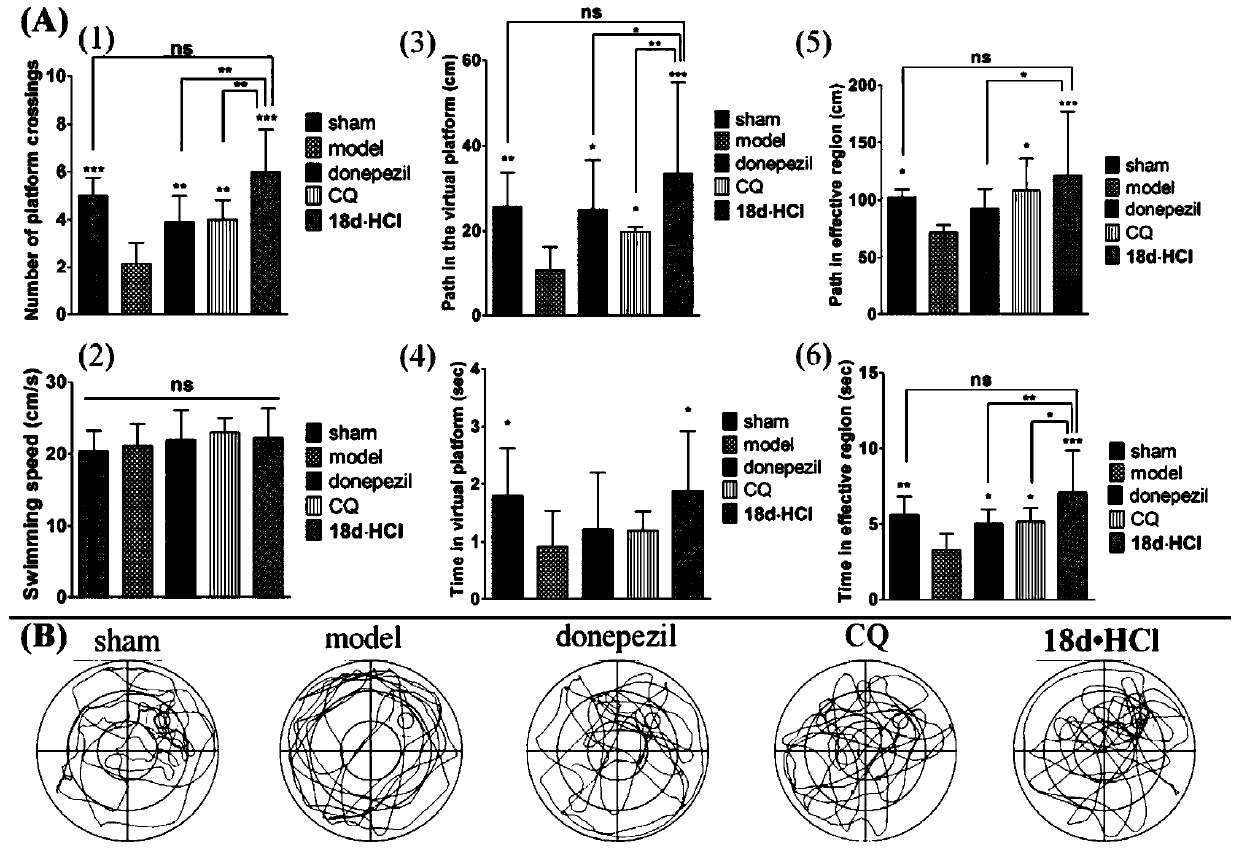
Benzofuran quinoline derivatives and their application in the preparation of medicines for treating Alzheimer's disease
A technology of benzofuran-quinoline and Alzheimer's disease, applied in antipyretics, drug combinations, nervous system diseases, etc., can solve problems such as inability to cure, achieve strong metal ion complexing ability, and inhibit nerves Inflammation, improved cognition and spatial memory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: 5-(5-hydroxybenzofuran-2-substituted)-8-hydroxyquinoline
[0036]
[0037] (1) Step 1: Synthesis of 5-chloromethyl-8-hydroxyquinoline hydrochloride
[0038]
[0039] Add 14.50g (100mmol) of 8-hydroxyquinoline, 16mL of concentrated hydrochloric acid, 16mL of 37% formaldehyde solution into a 100mL round-bottom bottle, pass hydrogen chloride gas under ice bath for 6h, stir at room temperature for 2h, filter with suction, wash with water, and dry in vacuo , 15.12 g of a yellow solid was obtained, and the yield was 78%.
[0040] (2) Step 2: Synthesis of 8-hydroxy-5-quinolinemethylphosphonodiethyl ester
[0041]
[0042]Add 13.55g (70mmol) 5-chloromethyl-8-hydroxyquinoline hydrochloride and 36mL triethyl phosphite to a 100mL round bottom bottle, react at 110°C for 6h, and distill off excess triethyl phosphite under reduced pressure The ester was separated by silica gel column chromatography and eluted with ethyl acetate to obtain 16.95 g of a light yello...
Embodiment 2
[0061] Example 2: 5-(5-hydroxybenzofuran-2-substituted)-7-chloro-8-hydroxyquinoline
[0062]
[0063] (1) Synthesis of 7-chloro-8-hydroxyl-5-quinolinemethylphosphonodiethyl ester
[0064]
[0065] Add 5 g of 8-hydroxy-5-quinolinemethylphosphonodiethyl ester and 30 mL of water into a 100 mL round bottom flask, add 1 NKOH (17 mL) solution, and add NaClO solution (15.3 mL, 7.5% excess) dropwise at room temperature. After the reaction was complete, dilute hydrochloric acid was added to adjust the pH to 6.0, and suction filtered to obtain 4.6 g of a white solid with a yield of 84%. R f =0.33 (CH 2 Cl 2 / CH 3 OH=20 / 1). 1 H NMR (400MHz, CDCl 3 )δ8.81(dd, J=4.1,1.0Hz,1H),8.44(dd,J=8.6,1.2Hz,1H),7.51(dd,J=8.6,4.2Hz,1H),7.45(d,J =3.8Hz,1H),4.05–3.93(m,4H),3.50(s,1H),3.44(s,1H),1.21(t,J=7.1Hz,6H).LC / MS(ESI):330.1 [M+H] + .
[0066] (2) Synthesis of 7-chloro 8-methoxyl-5-quinolinemethylphosphonodiethyl ester
[0067]
[0068] Add 2g of 7-chloro-8-hydroxy-5-quinolinemethy...
Embodiment 3
[0081] Example 3: 5-(5-hydroxybenzofuran-2-substituted)-7-iodo-8-hydroxyquinoline
[0082]
[0083] (1) Synthesis of 7-iodo-8-hydroxyl-5-quinolinemethylphosphonodiethyl ester
[0084]
[0085] Add 5g of 8-hydroxy-5-quinolinemethylphosphonodiethyl ester and 30mL of water into a 100mL round bottom bottle, add 1NKOH (40mL) solution, add the equivalent iodine and potassium iodide mixture in batches at room temperature. After the reaction was complete, dilute hydrochloric acid was added to adjust the pH to 6.0, and suction filtered to obtain a black solid, which was separated by silica gel column chromatography and eluted with ethyl acetate to obtain a brown solid. Yield 65%. R f =0.32 (CH 2 Cl 2 / CH 3 OH=20 / 1). 1 H NMR (400MHz, CDCl 3 )δ8.77(dd, J=4.2,1.2Hz,1H),8.43(dd,J=8.6,1.3Hz,1H),7.74(d,J=3.9Hz,1H),7.53(dd,J=8.6 ,4.2Hz,1H),4.10–3.90(m,4H),3.47(s,1H),3.42(s,1H),1.21(t,J=7.1Hz,6H).LC / MS(ESI):422.0 [M+H] + .
[0086] (2) Synthesis of 7-iodo8-methoxy-5-quinolinem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com