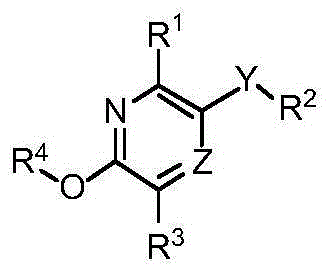
Substituted pyridine and pyrazine compounds as pde4 inhibitors
A technology for compounds and chemical entities, which can be used in in vivo radioactive agents, anti-inflammatory agents, drug combinations, etc., to solve problems such as limited usefulness and tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0708] Example 1. 5-({6-[3-(Difluoromethoxy)phenyl]-5-ethoxypyrazin-2-yl}methyl)pyrimidine-2-carbonitrile.
[0709]
[0710] Combine 5-(bromomethyl)-3-(3-(difluoromethoxy)phenyl)-2-ethoxypyrazine (Intermediate 1, 176.00 mg, 0.49 mmol), 5-( 4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)pyrimidine-2-carbonitrile (124.55mg, 0.54mmol), EtOH (2.45mL), Benzene (7.00mL), Pd(PPh 3 ) 4 (56.63mg, 0.05mmol) and NaHCO 3 Aqueous solution (1.38 mL, 1.15 mol / L, 1.59 mmol). The vial was sealed, purged with nitrogen and heated to 125°C under microwave conditions for 15 minutes. Use a pipette to remove water from the reaction, and via The crude reaction mixture was filtered and washed with EtOAc (3 x 5 mL). dry (Na 2 SO 4 ) combined organic layers, and the solvent was removed under reduced pressure. Purification (FCC, SiO 2 , 0-30%, EtOAc / hexanes) provided the title compound (100 mg, 53%) as a white solid. 1 HNMR (400MHz, CD 3 OD)δ8.94(s,2H),8.17(s,1H),7.95-7.90(m,1H),7.85(t,J=1.8...
Embodiment 2
[0711] Example 2. 2-Chloro-5-{[5-(3-chlorophenyl)-6-methoxypyridin-3-yl]methyl}pyrimidine.
[0712]
[0713] To 5-(bromomethyl)-3-(3-chlorophenyl)-2-methoxypyridine (intermediate 2,100mg, 0.321mmol), (2-chloropyrimidin-5-yl)boronic acid (76mg, 0.481 mmol) in ACN (3.2 mL) was added with NaHCO 3 (417mg, 1.282mmol) and PdCI 2(dppf)-DCM (23 mg, 0.032 mmol). The reaction was heated under microwave conditions at 120°C for 12 minutes. Water was removed from the reaction using a pipette, and via The crude reaction mixture was filtered and washed with EtOAc. dry (Na 2 SO 4 ) combined organics, filtered and concentrated on silica. Purification (FCC, SiO 2 , 30%-70% EtOAc / hexanes) provided the title compound (61 mg, 55%). 1 HNMR (400MHz, DMSO-d 6 )δ8.34(s, 2H), 8.11(d, J=2.0Hz, 1H), 7.70(d, J=2.0Hz, 1H), 7.58(t, J=1.8Hz, 1H), 7.53-7.36( m,3H), 3.83(s2H), 3.73(s,3H). [M+H] = 346.11.
Embodiment 3
[0714] Example 3. {2-[(5-{[5-(3-chlorophenyl)-6-methoxypyridin-3-yl]methyl}pyrimidin-2-yl)amino]ethyl}dimethyl amine.
[0715]
[0716] To 2-chloro-5-{[5-(3-chlorophenyl)-6-methoxypyridin-3-yl]methyl}pyrimidine (Example 2, 50.00mg, 0.14mmol) in ACN (1.44mL ) was added N1,N1-dimethylethane-1,2-diamine (0.03 mL, 0.29 mmol) and DIPEA (77.13 μL, 0.43 mmol). The reaction mixture was heated at 180°C for 15 minutes. EtOAc (5 mL) was added to the reaction mixture, and the reaction mixture was extracted with water (3x). dry (Na 2 SO 4 ) combined organic layers, and the solvent was removed under reduced pressure. Purification (FCC, SiO 2 , 0-15% MeOH / DCM) provided the title compound (15.6 mg, 28%). 1 HNMR (400MHz, DMSO-d 6 )δ8.21(s,2H),8.09(s,1H),7.67(brs,2H),7.58(s,1H),7.51-7.34(m,2H),6.79(brs,1H),3.84(s ,3H), 3.72(s,2H), 2.42-2.37(m,4H), 2.17(s,6H). [M+H] = 398.20.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com