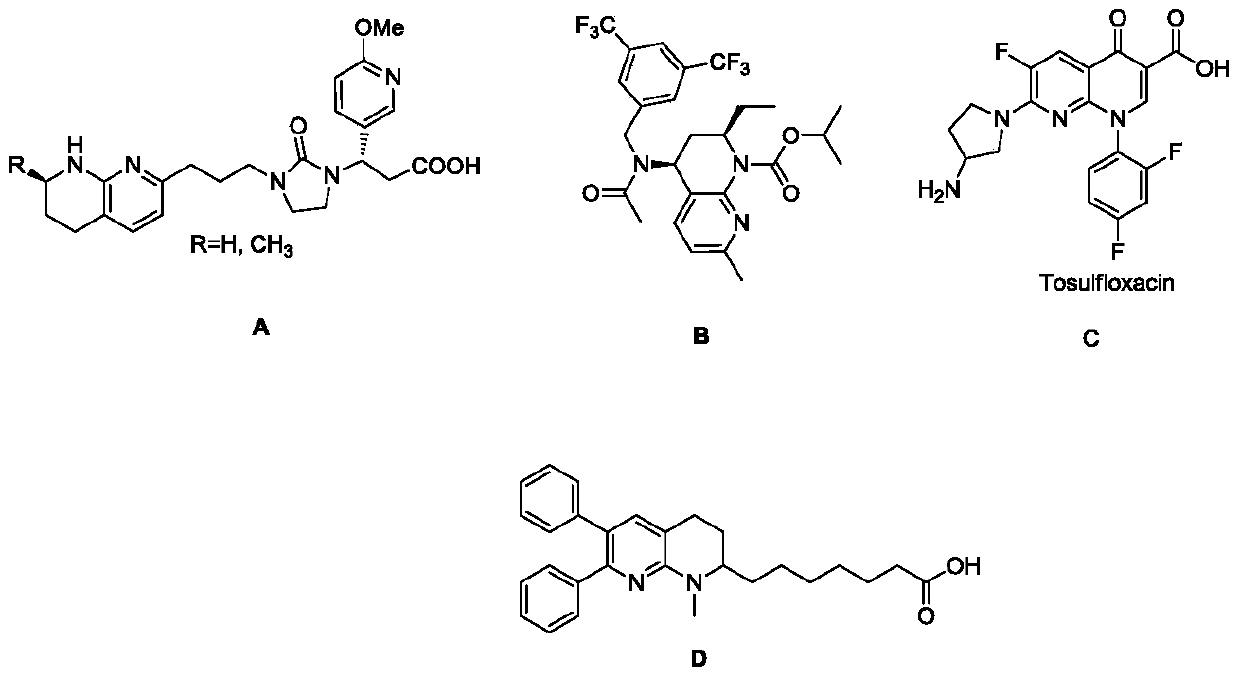
A kind of preparation method of tetrahydro-1,8-naphthyridine compound and the chiral product thereof
A compound and naphthyridine technology are applied in the preparation of tetrahydro 1,8-naphthyridine compounds and the field of the prepared chiral products, and can solve problems such as catalyst poisoning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
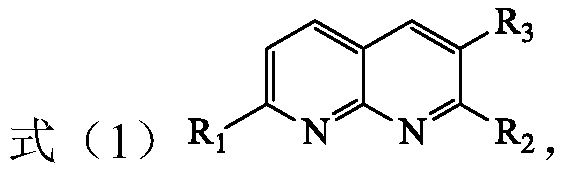
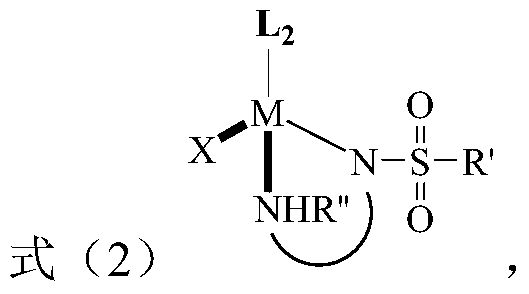
[0021] The present invention provides a method for preparing tetrahydro 1,8-naphthyridine compounds, wherein the method includes: in the presence of a chiral catalyst, the compound of the structure represented by formula (1) is subjected to an addition reaction with hydrogen, wherein , The chiral catalyst is a complex with the structure represented by formula (2);
[0022]
[0023] Where R 1 , R 2 And R 3 Each is independently hydrogen, substituted or unsubstituted C1-C10 alkyl, substituted or unsubstituted C3-C10 cycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted arylbenzyl Base, or R 2 And R 3 Linked to form a C5-C8-membered alkane ring, wherein the substituents in the substituted alkyl, substituted cycloalkyl, substituted aryl and substituted arylbenzyl are each independently selected from fluorine, chlorine, bromine, and nitro One or more of group, methyl group, methoxy group, trifluoromethyl group, hydroxyl group and acetamido group; and, R 1 Not ...
preparation example 1
[0137] (1) Dissolve (R,R)-1,2-diphenyl-ethylenediamine (20mmol, 24694 brand from Bailingwei Technology Co., Ltd.) in dichloromethane (30mL), and dissolve in dichloromethane (30mL) at 0℃ The p-methylphenylsulfonyl chloride (20mmol, purchased from Bailingwei Technology Co., Ltd. 283322 brand) in dichloroethane (30mL) was added dropwise to it (dropped within 30min), and the reaction was continued at 0°C for 1h, and then reduced pressure Rotary evaporation, the solid was separated and purified by column chromatography (the eluent was dichloromethane / methanol with a volume ratio of 10:1) to obtain 15 mmol of the formula (R, R)-(3-1-1-1) The chiral diamine shown has a yield of 75%. The identification data of the chiral diamine is: 1 H NMR(300MHz, CDCl 3 ):δ7.31(d,J=8.3Hz,2H), 7.18-7.09(m,10H), 6.97(d,J=8.3Hz,2H), 4.37(d,J=5.2Hz,1H), 4.12 (d,J=5.2Hz,1H),2.32(s,3H),1.49(br,3H); 13 C NMR(75MHz, CDCl 3 ): δ142.5, 139.2, 137.2, 129.1, 128.4, 128.2, 127.5, 127.4, 127.0, 126.9, 126.6, 63.2,...
preparation example 2
[0141] (1) According to the method of step (1) in Preparation Example 1, the difference is that methanesulfonyl chloride (20mmol, purchased from Alfa Aesar Chemical Co., Ltd. A13383 brand) is used instead of p-methylphenylsulfonyl chloride, and Reacted at 0°C for 1 hour to obtain 14.6mmol of the chiral diamine represented by the formula (R,R)-(3-1-1-2) with a yield of 73%. Identification data of the chiral diamine for: 1 H NMR(300MHz, CDCl 3 ): δ7.34-7.26 (m, 10H), 4.56 (d, J = 5.1 Hz, 2H), 4.21 (d, J = 5.1 Hz, 2H), 2.26 (s, 3H); 13 C NMR(75MHz, CDCl 3 ): δ141.9, 139.7, 128.7, 128.6, 127.9, 127.8, 126.9, 126.7, 63.4, 60.2, 40.7.
[0142] (2) According to the method of step (2) in Preparation Example 1, the difference is that the chiral diamine used is the chiral diamine represented by the above formula (R,R)-(3-1-1-2) (145mg, 0.5mmol), recrystallized to obtain 275mg of a red solid (ie the complex represented by formula (R, R)-4j);
[0143] (3) According to the method of step (3) i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com