Application of artesunate in preparation of medicine for treating idiopathic pulmonary fibrosis
A technology for pulmonary fibrosis and artesunate, applied in the field of biomedicine, can solve the problems of high cost, no public report of artesunate idiopathic pulmonary fibrosis drug, lack of donors, etc. Medicinal prospects, efficacy in inhibiting disease progression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
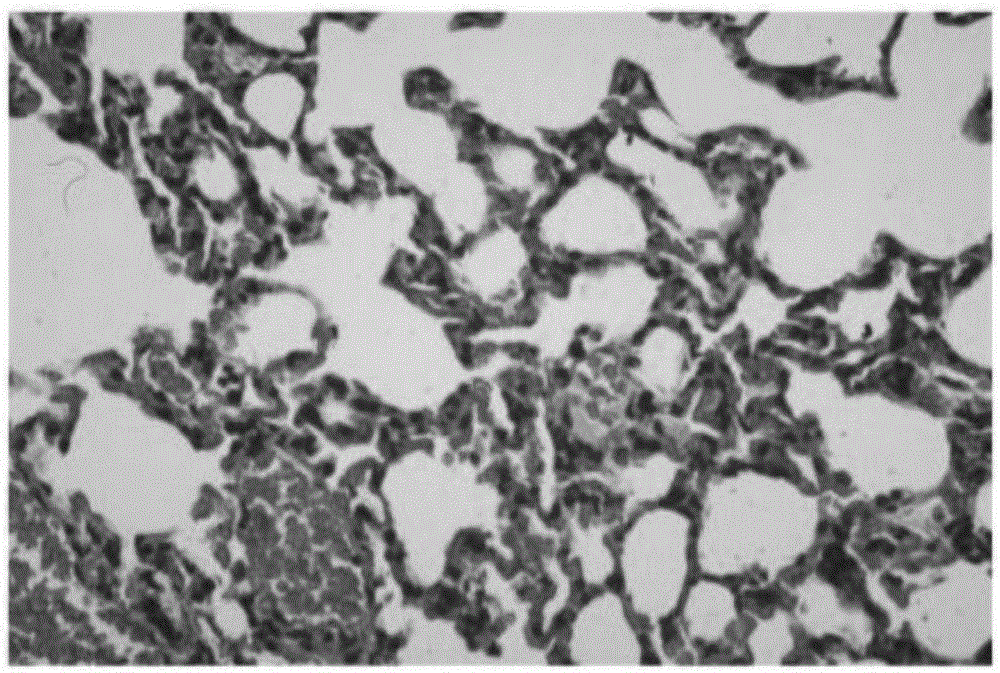
[0028] The principles and features of the present invention are described below in conjunction with the accompanying drawings, and the examples given are only used to explain the present invention, and are not intended to limit the scope of the present invention.
[0029] (1) Establishment of animal model of pulmonary fibrosis
[0030] Eighty male SD rats weighing 180-250 g were purchased from the SPF Animal Experiment Center of Guilin Medical College. They were randomly divided into normal saline control group (abbreviated as NS group), normal saline + bleomycin control group (abbreviated as BLM group), bleomycin + artesunate treatment group (abbreviated as AT group) and normal saline + Artemisia annua group. In the succinate control group (abbreviated as AC group), the rats in each group were randomly divided into 20 rats / group.
[0031] Normal saline control group (NS group): 0.3-0.4 mL of normal saline was injected into the trachea once, and 1 mL of normal saline was inje...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass volume concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com