Vortioxetine hydrobromide preparation method
A technology of vortioxetine and o-bromoiodobenzene, which is applied in the field of new antidepressant drugs, can solve the problems of unsuitability for industrial production, poor purity, long route, etc., and solve the problem of competing side reactions, reduce the number of impurities, and improve the yield and the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
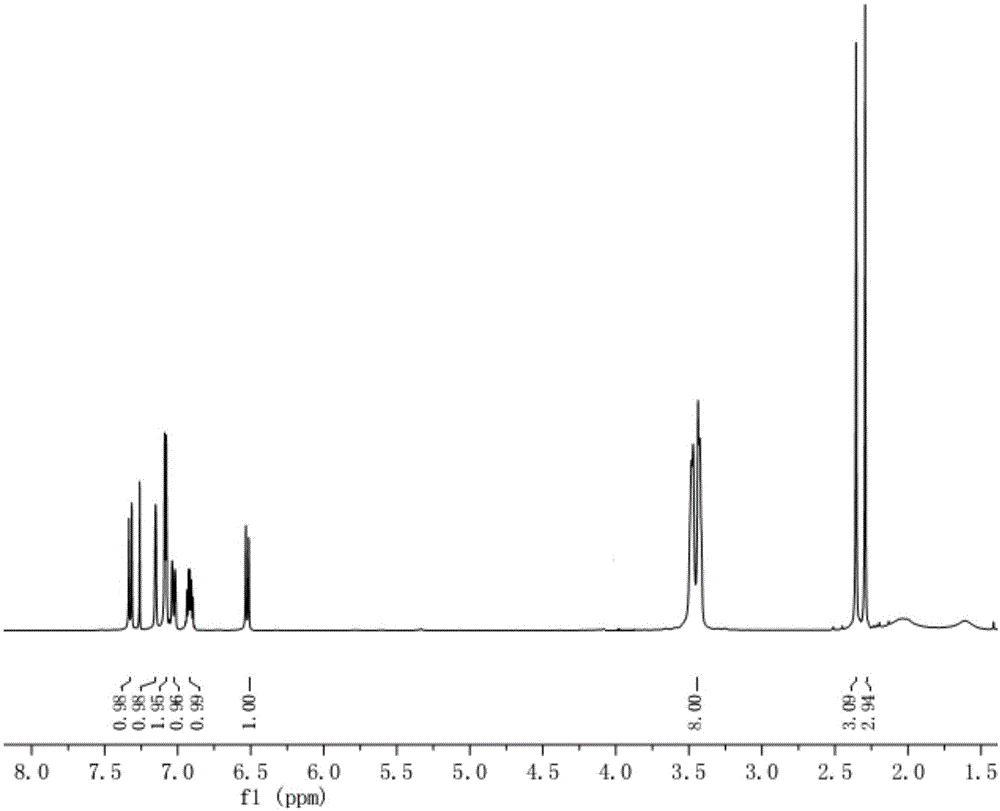
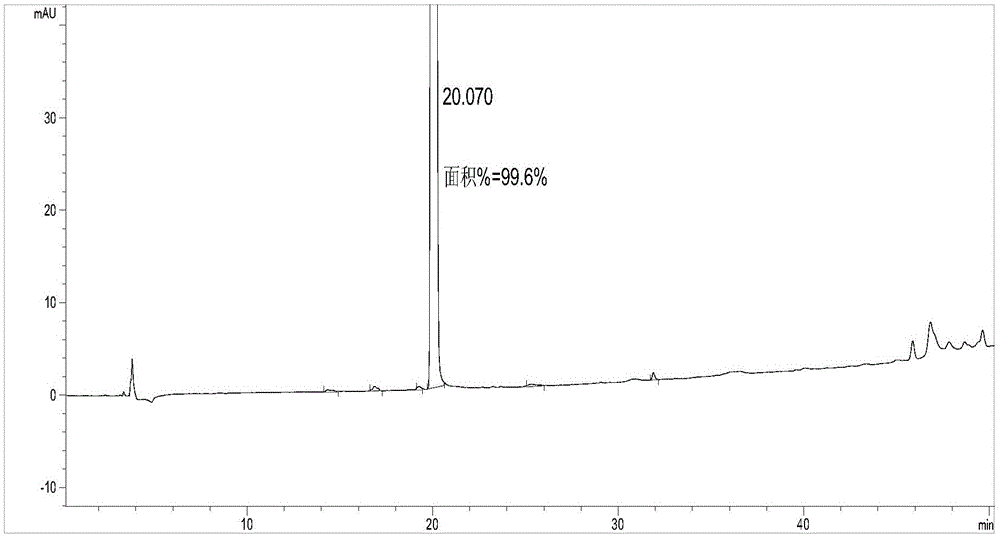
Image
Examples
Embodiment 1
[0051] The first step: the synthesis of 2-(2,4-dimethylphenylsulfanyl) bromobenzene (compound IV)
[0052] Into a 2L single-necked bottle, sequentially add 127.5g (0.45mol) of o-bromoiodobenzene, 68.4g (0.5mol) of 2,4-dimethylthiophenol, 4.3g (22.5mmol) of ketone iodide, and ethylene glycol 56.0g (0.9mol), isopropanol 1.1L, tripotassium phosphate 191.0g (0.9mol), nitrogen gas was introduced under normal pressure and room temperature under stirring, vacuum replaced three times, reacted at 80-90°C for 7-8h, stopped heating, and waited After the reaction solution was cooled to room temperature, the mother liquor was spin-dried at 60-70°C to obtain an oil. Add 1.5 L of dichloromethane to the oil, add 1 L of water, stir at room temperature for 10-20 minutes, filter with diatomaceous earth, and separate the mother liquor. 15% sodium chloride was washed 1 L x 2 times, washed 1 L x 1 time, dried over anhydrous magnesium sulfate for more than 4 hours, and DCM was spin-dried to obtain 1...
Embodiment 2
[0057] The first step: the synthesis of 2-(2,4-dimethylphenylsulfanyl) bromobenzene (compound IV)
[0058] Into a 1L single-necked bottle, sequentially add 28.3g (0.1mol) of o-bromoiodobenzene, 16.6g (0.12mol) of 2,4-dimethylthiophenol, 1.9g (10mmol) of ketone iodide, and 12.4g of ethylene glycol g (0.2mol), 250ml of isopropanol, 42.4g (0.2mol) of tripotassium phosphate, nitrogen gas was introduced under normal pressure and room temperature, and replaced by vacuum three times, reacted at 80-90°C for 7-8h, stopped heating, and the reaction solution was After cooling to room temperature, the mother liquor was spin-dried at 60-70°C to obtain an oil. Add 300ml of dichloromethane to the oil, add 200ml of water, stir at room temperature for 10-20min, filter with diatomaceous earth, separate the mother liquor, and add 15% chlorine Wash with sodium chloride water 200ml x 2 times, water 200nl x 1 time, dry over anhydrous magnesium sulfate for more than 4h, and spin dry DCM to obtain 30...
Embodiment 3
[0062] The first step: the synthesis of 2-(2,4-dimethylphenylsulfanyl) bromobenzene (compound IV)
[0063] Into a 1L single-necked bottle, add 28.3g (0.1mol) of o-bromoiodobenzene, 11.1g (0.08mol) of 2,4-dimethylthiophenol, 0.6g (3mmol) of ketone iodide, and 9.3 g (0.15mol), 250ml of isopropanol, 31.8g (0.15mol) of tripotassium phosphate, nitrogen gas was introduced under normal pressure and room temperature stirring, vacuum replacement was carried out three times, reaction was carried out at 60-70°C for 10h, heating was stopped, and the mother liquor was spin-dried to obtain Oil, add 300ml of dichloromethane to the oil, add 200ml of water, stir at room temperature for 10-20min, filter with diatomaceous earth, separate the mother liquor, wash with 15% sodium chloride 200ml x 2 times, wash 200nl x 1 time , dried over anhydrous magnesium sulfate for more than 4 hours, and spin-dried DCM to obtain 24.5 g of light yellow oil (yield: 100%).
[0064] The second step: the synthesis ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap