Synthetic linear apelin mimetics for the treatment of heart failure
A technology of D-L, X1-R-P-R-X5-S-X7-K-G-P-X11-X12-X13, applied in the field of new compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0102] In embodiment 1, the present invention thus provides a peptide or polypeptide of formula (I):
[0103] X1-X2-X3-R-X5-X6-X7-X8-X9-X10-X11-X12-X13
[0104] I
[0105] among them:
[0106] X1 is the N-terminus of the polypeptide and does not exist, is Q, A or pE;
[0107] X2 is R or r;
[0108] X3 is P or 4-PhP;
[0109] X5 is L, Cha, D-L, F, Y, Y(Bzl), 3,4-Cl2-F or NaI;
[0110] X6 is D-amino acid, S or A;
[0111] X7 is D-amino acid, L, H or Aib; and at least one of X6 and X7 is D-amino acid or Aib;
[0112] X8 is K, k, Q or E;
[0113] X9 is G or D;
[0114] X10 is P or pipecolic acid;
[0115] X11 is D-Nle, Nle, f or D-Nva;
[0116] X12 does not exist, is a P or D-amino acid;
[0117] X13 is C-terminal and does not exist, is an F or D-amino acid; and at least one of X11, X12 and X13 is a D-amino acid;
[0118] among them:
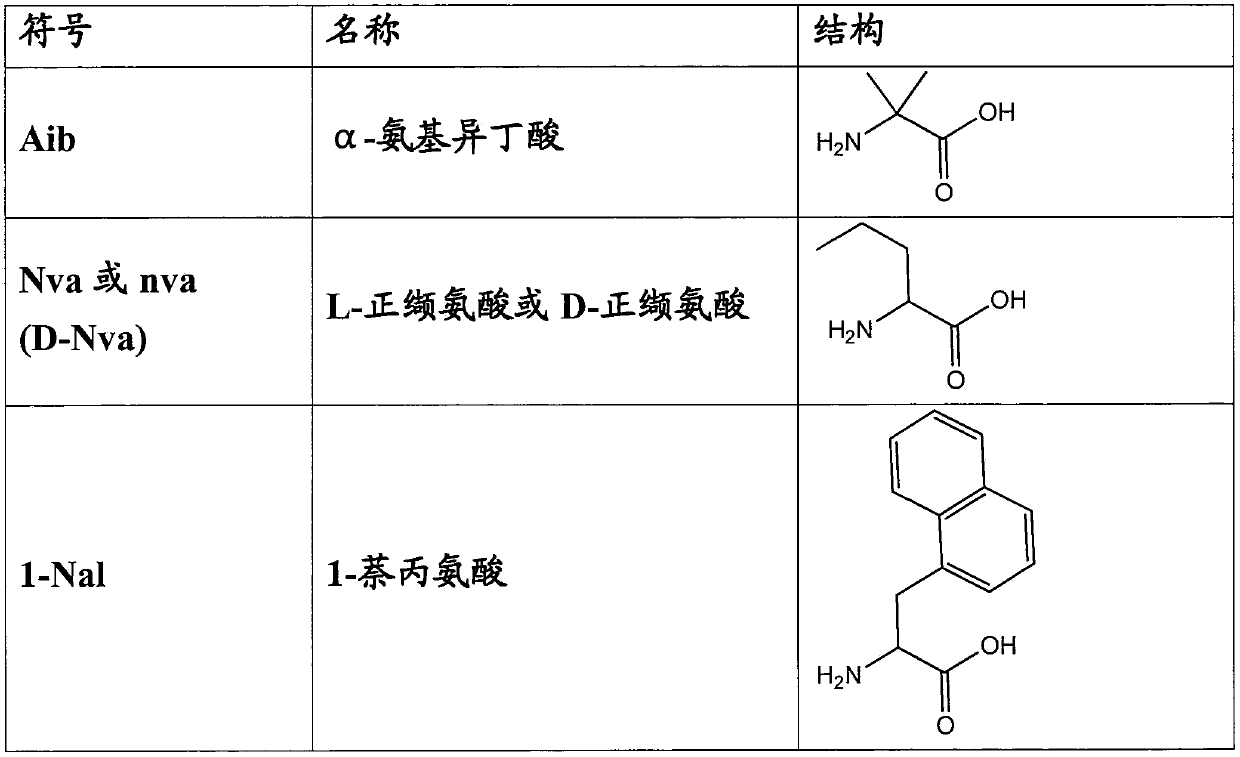
[0119] Nle is L-norleucine;
[0120] D-Nle is D-norleucine;
[0121] Nal is L-(naphthyl)alanine;
[0122] D-Nva is D-Norvalin...
Embodiment approach 1a
[0131] In embodiment 1a, the present invention thus provides peptide or polypeptide formula (IA):
[0132] X1-X2-X3-R-X5-X6-X7-X8-G-X10-X11-X12-X13
[0133] IA
[0134] among them:
[0135] X1 is the N-terminus of the polypeptide and does not exist, is Q, A or pE;
[0136] X2 is R or r;
[0137] X3 is P or 4-PhP;
[0138] X5 is L, Cha, D-L, F, Y, Y(Bzl), 3,4-Cl2-F or 2-NaI;
[0139] X6 is D-amino acid or S;
[0140] X7 is D-amino acid, H or Aib; and at least one of X6 and X7 is D-amino acid or Aib;
[0141] X8 is K or k;
[0142] X10 is P or pipecolic acid;
[0143] X11 is D-Nle or Nle;
[0144] X12 does not exist, is a P or D-amino acid;
[0145] X13 is C-terminal and does not exist, is an F or D-amino acid; and at least one of X11, X12 and X13 is a D-amino acid;
[0146] Or an amide, ester or salt of the polypeptide; or a polypeptide substantially equivalent thereto.
[0147] In embodiment 1b, the present invention relates to a peptide or polypeptide of...
Embodiment approach 8
[0156] In embodiment 8, the present invention relates to the polypeptide of any one of embodiments 1 (1A or 1B)-7, having the following formula II:
[0157] X1-R-P-R-X5-a-X7-X8-G-P-X11-X12-X13
[0158] II
[0159] Or the amide, ester or salt of the polypeptide.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com