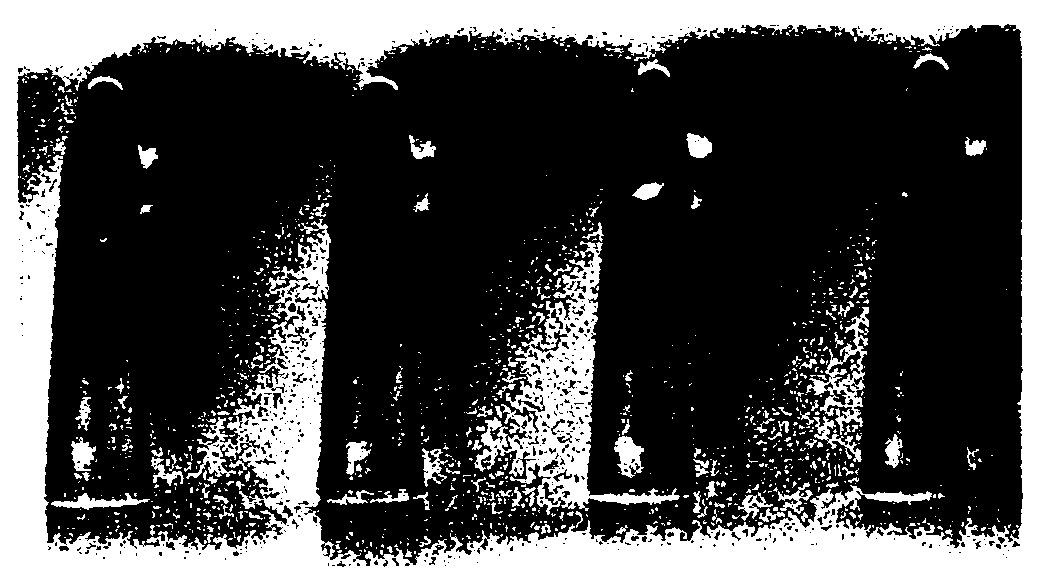A drug-retaining microneedle matrix and its manufacturing method
A micro-needle and drug technology, applied in the directions of micro-needle, drug devices, needles, etc., can solve the problems of no indication that the drug is peeled off, the quantitative drug delivery effect is not ideal, and it is cumbersome.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
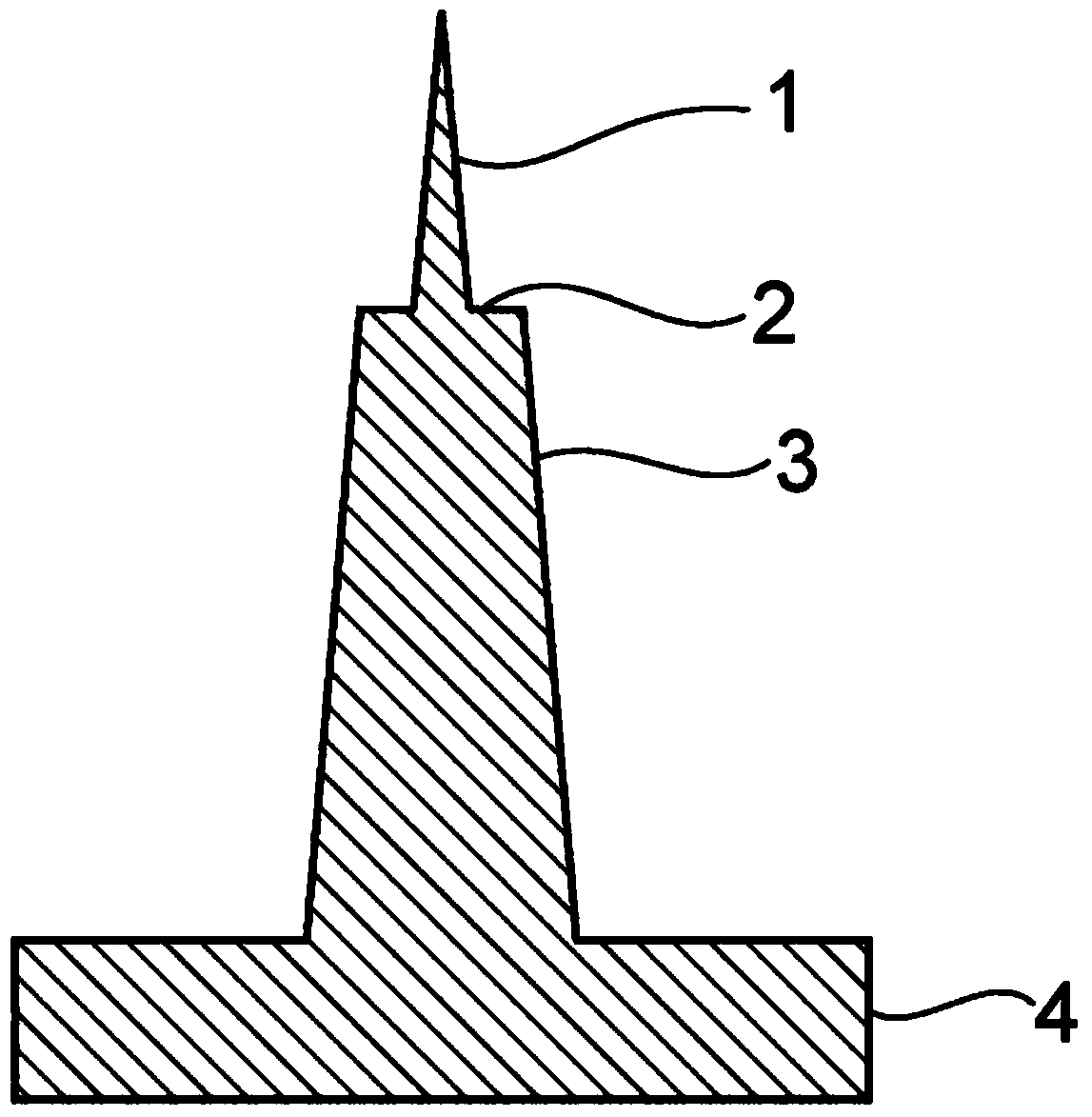
[0065] Using nylon 12 as material, the microneedle matrix with microneedles with stepped parts and the microneedle matrix with microneedles without stepped parts were manufactured by injection molding. figure 1 It shows the structure of one microneedle with a stepped part among the plurality of microneedles arranged in the microneedle matrix. In the figure, 1 is a tip portion, 2 is a step portion, 3 is a root portion, and 4 is a base plate. The tip length is 200 μm, the root length is 430 μm, the step edge size is 30 μm, and the needle gap is 400 μm.
[0066] Partially immerse the microneedle tips of two microneedle arrays (diameter 1 cm) from the tip to 100 μm in an aqueous solution of hyaluronic acid (FCH-80LE Kikkoman-Biochemifa Co., Ltd.) and blue pigment (Blue No. 1, NacalaiTesque Co., Ltd.) middle. Blue pigments are used instead of medicines to easily observe the adhesion state of medicines under microscope observation.
[0067] For microneedles with steps, after the ...
Embodiment 2
[0071] Using nylon 12 (L1640Daicel-Degussa Co., Ltd.) as raw material, the microneedle matrix with steps was prepared by injection molding. The dimensions of the microneedles are as follows: tip length 270 μm, tip upper diameter 20 μm, tip lower diameter 60 μm, root length 160 μm, root lower diameter 140 μm, root upper diameter 130 μm, edge size 35 μm, needle gap 400 μm. Among them, the upper part and the lower part are based on the state where the tip part is placed on the top and the root part is placed on the bottom. The diameter of the microneedle matrix is 1 cm. image 3 A micrograph showing the formed microneedle. In addition, microneedles with stepped portions were produced with the same tip size and needle gap, and changed the root size so that the edge size was 50 and 100 μm, respectively.
[0072] The effectiveness of the step was evaluated by changing the size of the edge of the step.
[0073] When the size of the edge portion of the step portion is smaller tha...
Embodiment 3
[0079] Polyglycolic acid (Kuredux, Kureha Co., Ltd.) was used as a raw material, and injection molding was performed under the same conditions as in Example 2 except that the injection temperature was 260° C., to obtain a microneedle with a stepped portion with a step edge size of 35 μm. Using this microneedle, a 90 μm tip portion of the microneedle was immersed in an aqueous solution containing 10% of hydroxypropylcellulose (HPC-L, Soda Co., Ltd.) and 0.1% of a red pigment (Red No. 102, Nacalai Tesque Co., Ltd.). After lifting and drying and dipping and drying again, the coating state of the red pigment on the tip of the needle was observed with a solid microscope, and it was found that the red pigment stopped at the step.
[0080] This result shows that the result of Example 2 is not unique to the blue pigment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com