Synthesis method and application of thiopheneamide-substituted chiral phosphine ferrocene catalyst
A synthesis method and technology of thiophene amide, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., to achieve mild reaction conditions, good stability, and low dosage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
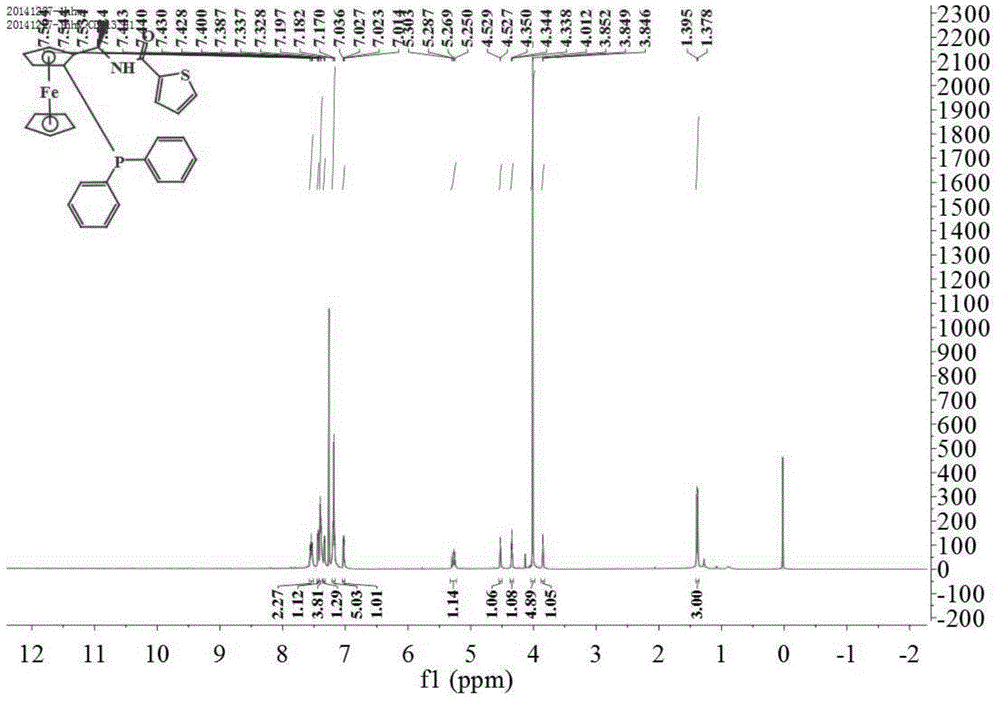
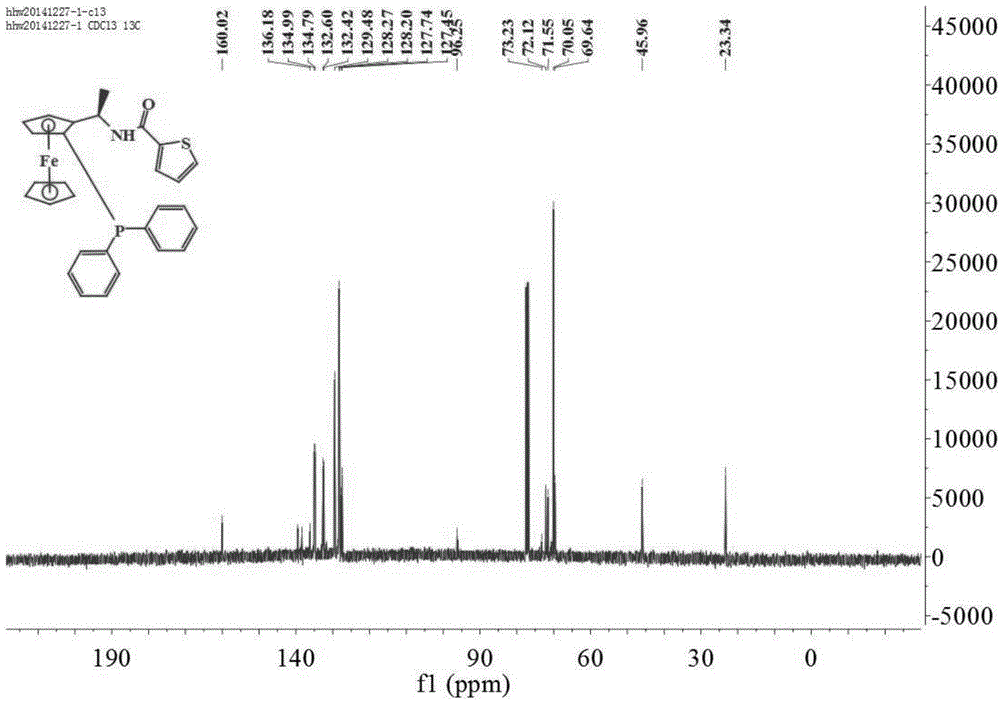
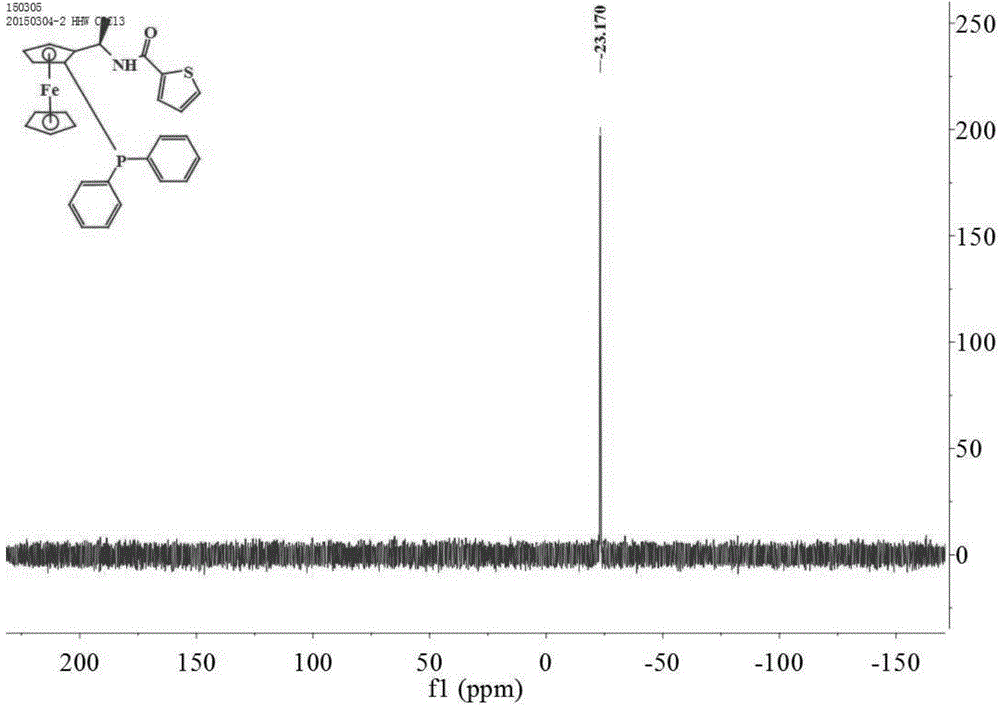
[0047] Add thiophene-2-carboxylic acid (0.096g, 0.75mmol), DCC (0.155g, 0.75mmol), dichloromethane (10mL) and (1S)-diphenylphosphino-(2R) into a 50mL two-necked flask successively. -Aminoethylferrocene(II) (0.207g, 0.5mmol), react at 20°C for 2 hours, add 15mL of water and stir for 30 minutes, filter with suction, pour the filtrate into a separatory funnel to separate the organic layer and the water layer Extracted 3 times with dichloromethane, washed 3 times with saturated brine, combined organic phase, anhydrous Na 2 SO 4 Drying and concentration under reduced pressure gave the crude product, which was subjected to column chromatography (petroleum ether: ethyl acetate = 15:1, V:V) to obtain 0.227 g of a yellow solid with a yield of 86.8%, m.p.70.1-71.4°C; [α] 20 D =-344.1°(c 1.0, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 )δ7.57–7.51(m,2H),7.44(dd,J=4.0,1.2Hz,1H),7.40(dd,J=5.2,1.2Hz,4H),7.33(dd,J=0.8,2.8Hz ,1H),7.21–7.16(m,5H),7.02(dd,J=5.2,3.6Hz,1H),5.27(m,1H),4.53(d,J=0.8Hz,...
Embodiment 2
[0049] Add thiophene-2-carboxylic acid (0.096g, 0.75mmol), DCC (0.103g, 0.5mmol) and anhydrous dichloromethane (5mL) successively into a 50mL two-necked flask, place in an ice bath, stir magnetically, and protect with nitrogen , the system was white suspension. After the temperature of the system was constant, a mixture of (1S)-diphenylphosphino-(2R)-aminoethylferrocene(II) (0.207g, 0.5mmol) and 5mL of anhydrous dichloromethane was slowly added dropwise, After dripping, move the reaction bottle to 30°C for reaction, TLC detects the reaction until the raw material (II) disappears, add 15mL water to quench the reaction, filter with suction, separate the white solid, pour the filtrate into the separatory funnel to separate the organic layer, water layer was extracted 3 times with dichloromethane, washed 3 times with saturated brine, and the organic phases were combined, anhydrous Na 2 SO 4 Drying; filtration and rotary evaporation to obtain the crude product, which was subjecte...
Embodiment 3
[0051] Add thiophene-2-carboxylic acid (0.096g, 0.75mmol), DCC (0.206g, 1.0mmol) and anhydrous dichloromethane (5mL) successively into a 50mL two-necked flask, place in an ice bath, stir magnetically, and protect with nitrogen . After the temperature of the system was constant, a mixture of (1S)-diphenylphosphino-(2R)-aminoethylferrocene(II) (0.207g, 0.5mmol) and 5mL of anhydrous dichloromethane was slowly added dropwise, After dripping, move the reaction bottle to 20°C for reaction, TLC detects the reaction until the raw material (II) disappears, add 15mL water to quench the reaction, filter with suction, separate the white solid, pour the filtrate into the separatory funnel to separate the organic layer, water layer was extracted 3 times with dichloromethane, washed 3 times with saturated brine, and the organic phases were combined, anhydrous Na 2 SO 4 Drying; the crude product was obtained by suction filtration and rotary evaporation, and 0.209 g of a yellow solid was obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com