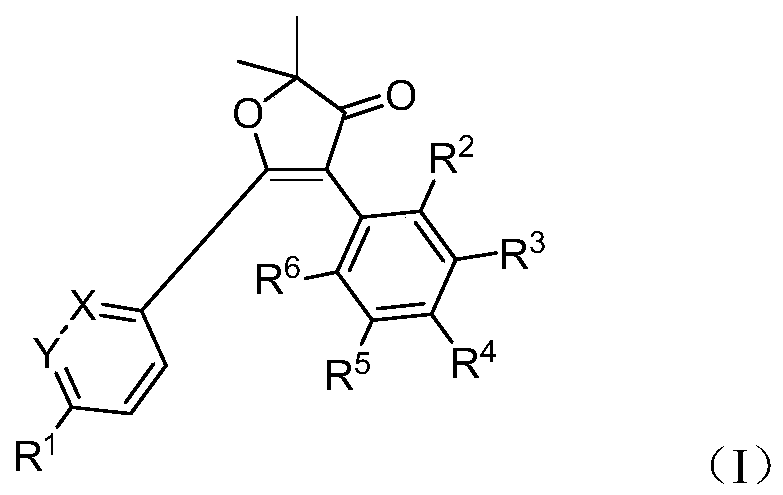
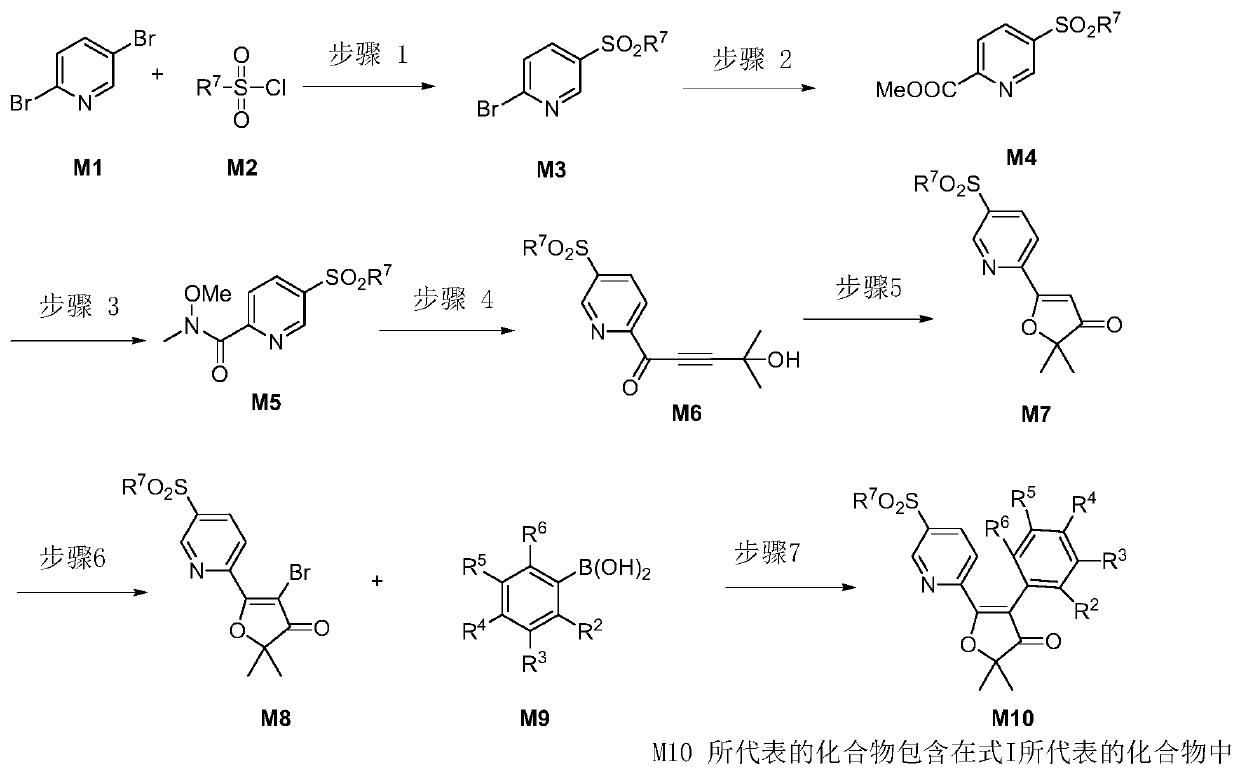
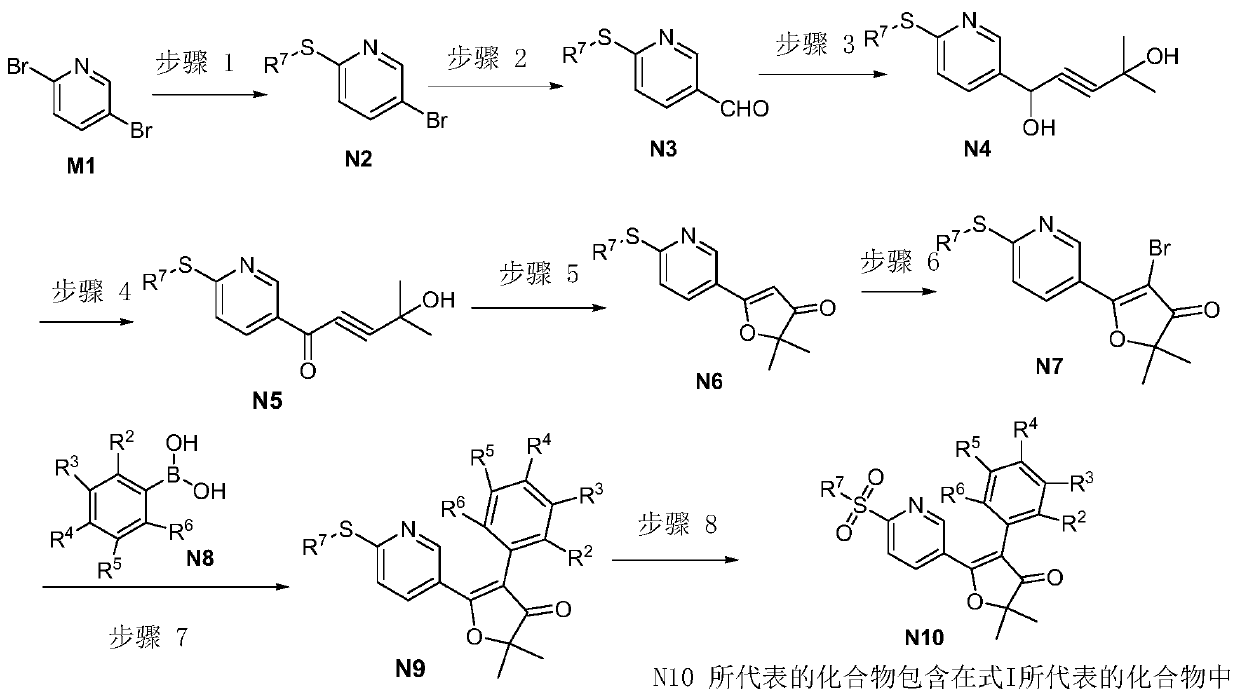
Furanone derivatives, preparation methods and uses
A kind of technology of furanone and compound, applied in the field of furanone derivative, preparation and use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0165] Example 1: Preparation of 2,2-dimethyl-5-[5-(methylsulfonyl)-2-pyridyl]-4-benzo-3(2H)-furanone Prepare (Compound A-001).
[0166]
[0167] Step 1: Preparation of 2-bromo-5-(methylsulfonyl)pyridine (compound 2)
[0168] 2,5-Dibromopyridine (50 g, 211 mmol) was dissolved in 175 mL THF, and cooled to 0° C. in an ice bath. Isopropylmagnesium chloride (2M THF solution, 274mmol) was slowly added to the above solution, and the temperature was controlled not to exceed 8°C. The reaction mixture was stirred at 0°C for 45 minutes and then cooled to minus 15°C. The THF (40 mL) solution of methanesulfonyl chloride was slowly added to the above reaction solution, the temperature was controlled not to exceed 5°C, and the solution was stirred for 30 minutes after dropping, and then water was added to separate the layers. The aqueous phase is extracted twice with methyl tert-butyl ether. The organic phases were combined and washed with water. Concentrated under reduced press...
Embodiment 2 to 28
[0182] Examples 2 to 28 can all be prepared by referring to the method of Example 1, and can also be prepared by other methods, which should not be construed as a limitation of the present invention. The corresponding compounds are shown in Tables 1-1 and 1-2, and the structure confirmation data of the compounds are shown in Table 1-3.
[0183] The chemical structural formula of table 1-1 embodiment 1-28
[0184]
[0185]
[0186] Table 1-2 Chemical names of A-001~A-028
[0187]
[0188]
[0189] The structural confirmation data of some compounds in Table 1-3 Examples 2-28
[0190]
Embodiment 29
[0191]Example 29: Preparation of 2,2-dimethyl-5-[6-(methylsulfonyl)-3-pyridine]-4-benzo-3(2H)-furanone (Compound B-001)
[0192]
[0193] 5-Bromo-2-methylthiopyridine (Compound S1)
[0194] 2,5-Dibromopyridine (35 g, 0.15 mol) was dissolved in 50 mL DMF. In the above solution, add 150mLNaSCH 3 aqueous solution (20%, 0.43mol). The reaction mixture was heated to 85°C for 24 hours. After cooling, the mixture was washed with CH 2 Cl 2 extraction. The organic phase was washed with saturated brine. After removing the solvent, the crude product was purified by column chromatography to obtain compound S1 (15 g, 50%, white solid). 1 H-NMR (300MHz, CDCl 3 ):δ8.49(s,1H),7.597(d,1H),7.280(d,1H),2.530(s,3H); LCMS[M+H] + :204; Purity (LCMS)>95%.
[0195] 6-Methylthio-3-formylpyridine (Compound S2)
[0196] Compound S1 was dissolved in 20 mL THF and cooled to -78 °C. Add n-BuLi (2 mL, 10.3 mmol, 2.5 M) slowly to the above solution, and stir for 2 hours. Then DMF (0.9 g, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com