Novel orally bioavailable breathing control modulating compounds, and methods of using same
A compound and chemical bond technology, applied in the direction of active ingredients of heterocyclic compounds, drug combinations, respiratory diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
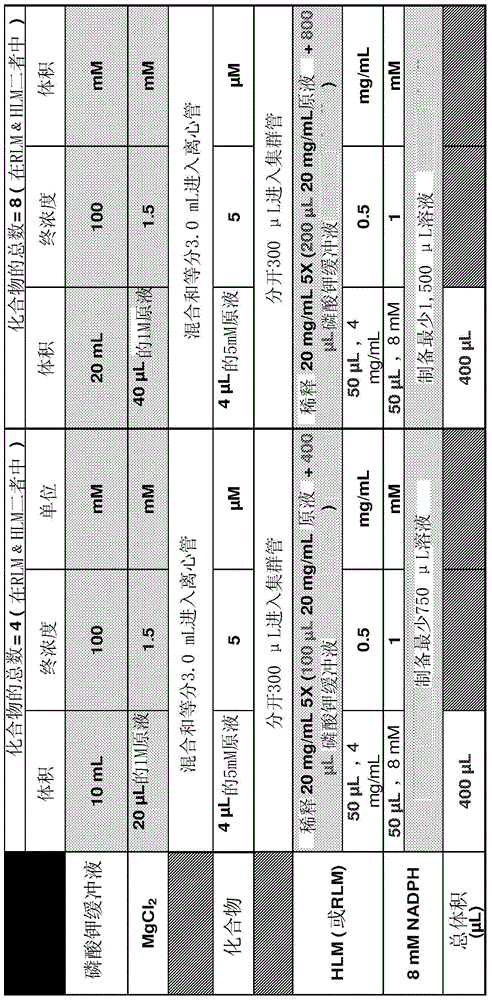
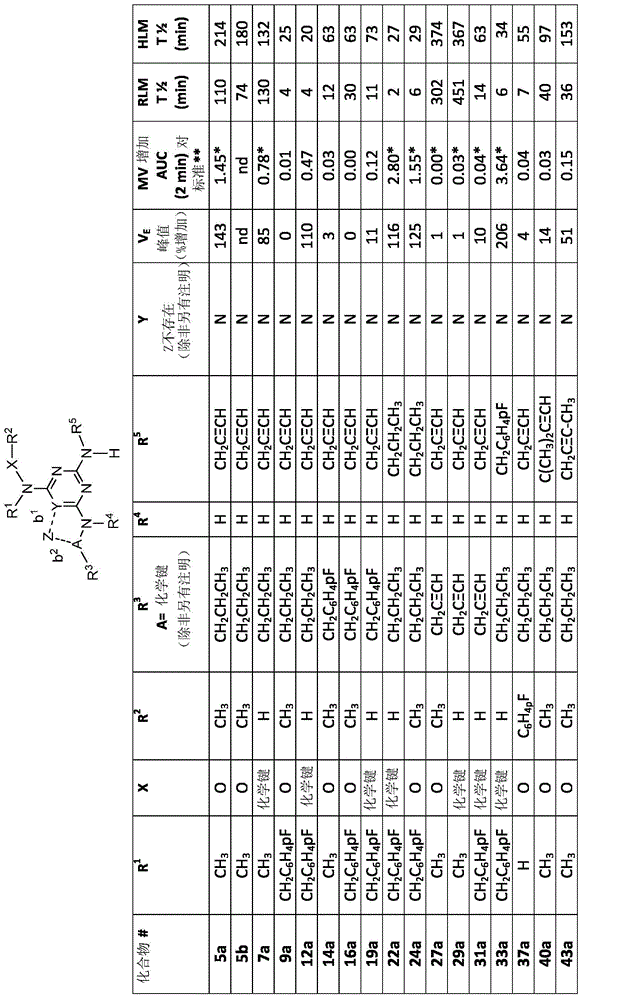
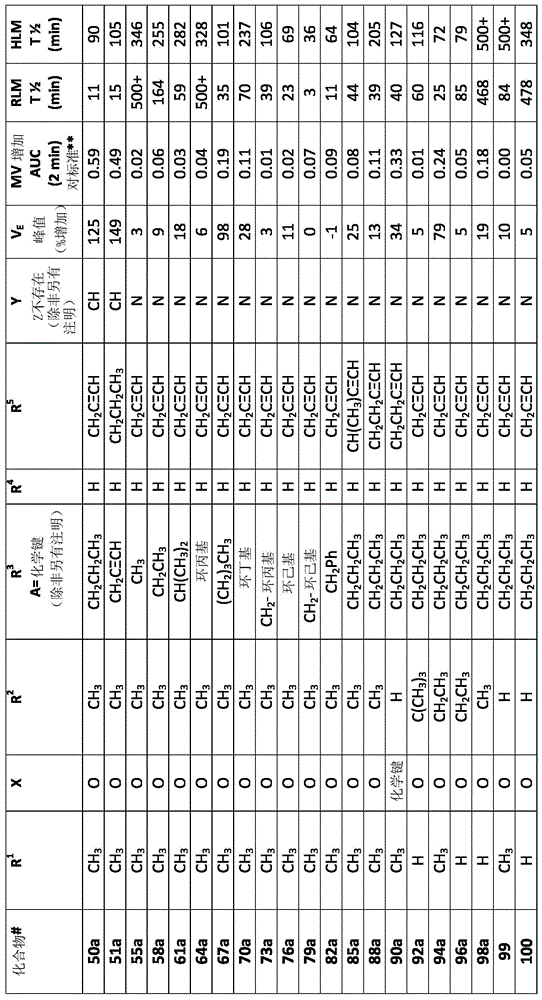
[0544] Example 1: O, N-dimethyl-N-[4(n-propylamino)-6-(prop-2-ynylamino)-[1,3,5]triazin-2-yl]- Hydroxylamine (4), and corresponding salts: hydrochloride (5a) and bisulfate (5b) (Scheme 10)
Embodiment 1A
[0545] Example 1A, stage 1: 2,4-dichloro-N-(6-n-propylamino)-[1,3,5]triazine (2); process and purity Method 3M-8 (In-Process and Purity Analysis, Method 3M-D):
[0546] To a 2 liter jacketed glass reactor with bottom drain valve, stirrer (three-bladed impeller), thermometer and dropping funnel (with pressure equalizing arm) was charged powdered cyanuric chloride (1) (120 g, 0.651 mol , 1 equiv) and THF (540 mL). The temperature in the jacket was set at -25°C.
[0547] Separately, n-propylamine (53.4 mL, 0.651 mol, 1 eq) and DIPEA (113.3 mL, 0.651 mol, 1 eq) were dissolved in THF (960 mL). This mixture was added dropwise to the stirred solution of (1) within 4 h at -25°C. After this time, the reaction mixture was allowed to warm to room temperature and stirred for 16 h. The volatiles were removed in vacuo and the resulting oily residue was partitioned between EtOAc (1000 mL) and water (300 mL). The organic layer was washed with water (2x300 mL), then brine solution (500...
Embodiment 1B
[0548] Example 1B, Stage 2: 6-Chloro-N 2 -(prop-2-ynylamino)-N 4 -n-Propylamino-1,3,5-triazine (3):
[0549] 4,6-Dichloro-[1,3,5]triazin-2-yl)-n-propyl-amine (2) (3.00g, 14.49mmol), propargylamine hydrochloride (1.46g, 15.94mmol ) and N,N-diisopropylethylamine (5.3 mL, 31.88 mmol) in 1,4-dioxane (25 mL) was stirred at 55° C. for 2 h. The mixture was cooled to room temperature. The resulting precipitate was filtered, washed with water and dried to give 6-chloro-N 2 -(prop-2-ynylamino)-N 4 - n-Propylamino-1,3,5-triazine-2,4-diamine (3) (2.98 g, 91%). 400MHz 1 H NMR (DMSO-d 6 , ppm): δ8.16-7.83 (2H, m), 4.01-3.93 (2H, m), 3.22-3.08 (2H, m), 3.08-3.03 (1H, m), 1.57-1.43 (2H, m) , 0.90-0.81 (3H, m). ESI-MS (m / z): 226, 228 [M+H] + .
[0550] Example 1C, Stage 3, Method 1: O,N-Dimethyl-N-[4-(n-propylamino)-6-(prop-2-ynylamino)-[1,3,5] Triazin-2-yl]-hydroxylamine (4); Process and Purity Method 3M-D:
[0551] 6-Chloro-N 2 -(prop-2-ynylamino)-N 4 - n-propylam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com