Detection reagent for prognosis of ZFP36 prostatic cancer and kit of detection reagent
A detection kit, a technology for prostate cancer, applied in the biological field, can solve problems such as insufficient specificity and increased PSA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
[0036] Experimental example: Selection experiment of markers indicative of tumor inflammatory state in prostate cancer
[0037] One: Bioinformatics data mining
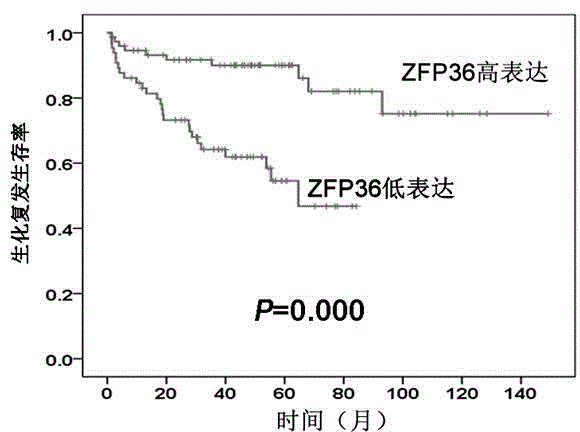
[0038] 1. Using the gene chip microarray data set (Taylor dataset), SPSS 13.0 was used to analyze the expression level of ZFP36 in 150 prostate cancer tissues. The results found that the expression level of ZFP36 was the same as that of serum PSA (P < 0.001), Gleason score (P < 0.001), pathological stage (P = 0.016), metastasis (P < 0.001) and biochemical recurrence (P < 0.001) were significantly correlated, that is, patients with low serum PSA, low Gleason score, low pathological stage, no metastasis, and no biochemical recurrence ZFP36 expression was higher in vivo (see Table 1). Therefore, studies have shown that ZFP36 is involved in the complex evolution process of prostate cancer and can be used as a molecular marker for judging the inflammatory state of prostate cancer.
[0039]
[0040] 2. The results sho...
Embodiment 1
[0053] Reagent name content (1) H 2 o 2 1-1.5ml (2) Non-immune sheep serum working solution 1-1.5ml (3) Primary antibody: rabbit polyclonal antibody against ZFP36 2.5-20ul (4) Secondary antibody: biotin-labeled goat anti-rabbit IgG working solution 1ml (5) Streptavidin-peroxidase solution 1ml (6) DAB developer 2-3ml
[0054] The method of using this kit is as follows:
[0055] (1) Place the slices that have been dewaxed, hydrated, and antigen-retrieved in the incubation box, and circle the tissue with a water-blocking pen to prevent the reagents from dripping from spreading, and absorb the water on the slices with absorbent paper. , do not touch the tissue, add H 2 o 2 Cover the tissue with 50ul and incubate at room temperature for 30min to block the activity of endogenous peroxidase;
[0056] (2) Rinse with PBS for 3 minutes, repeat 3 times;
[0057] (3) Slightly absorb the PBS solution on the slice with absorbent pap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com