Preparation methods of N-phenylacetyl-L-proline, and N-(1-(phenylacetyl)-L-prolyl)glycine ethyl ester
A technology of glycine ethyl ester and proline, applied in organic chemistry, peptides, etc., can solve the problems of unfavorable industrial production, low synthesis content, and low synthesis efficiency, and achieve high preparation efficiency, simple operation, and high preparation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation method of embodiment 1N-phenylacetyl-L-proline
[0032] Put 57.5kg of L-proline into the reaction kettle, then put in 250L of 2mol / l NaOH solution, stir for 30 minutes, the temperature is controlled at -10 to -5°C, and then double dropwise add 66L of phenylacetyl chloride and 125L of 4mol / l NaOH Liquid, the temperature is controlled at -10 to -5°C, after the dropwise addition, keep warm for 15 minutes, after the end, extract with 300L ethyl acetate, separate layers, remove the ethyl acetate layer, adjust the pH to 3 with 2mol / L hydrochloric acid, and then use 300L dichloromethane extraction, partition water layer, dichloromethane layer with 20kg anhydrous NaSO Dry, filter, filtrate decompression distillation, obtain oily matter 62kg, after cooling, N-phenylacetyl-L-proline is obtained, and its purity is 99.6%.
Embodiment 2
[0033] The preparation method of embodiment 2N-phenylacetyl-L-proline
[0034] Put 50kg of L-proline into the reaction kettle, then put into 250L 2mol / l potassium hydroxide solution, stir for 30 minutes, the temperature is controlled at -10 to -5°C, then double dropwise add 56L of phenylacetyl chloride and 125L of 4mol / l Potassium hydroxide solution, the temperature is controlled at -10 to -5°C, after the dropwise addition, keep warm for 15 minutes, after the end, extract with 300L ethyl acetate, separate layers, remove the ethyl acetate layer, and adjust the pH to 1.2 with 2mol / L hydrochloric acid , then extracted with 300L dichloromethane, partitioned the water layer, and the dichloromethane layer was dried with 20kg of anhydrous NaSO4, filtered, and the filtrate was distilled under reduced pressure to obtain 62kg of oily matter, and after cooling, N-phenylacetyl-L-proline was obtained. The purity is 99.7%.
Embodiment 3
[0035] The preparation method of embodiment 3N-phenylacetyl-L-proline
[0036] Put 57.5kg of L-proline into the reaction kettle, then put into 250L 2mol / l potassium hydroxide solution, stir for 30 minutes, control the temperature at -10 to -5°C, then add dropwise 60L of phenylacetyl chloride and 125L of 4mol / l Potassium hydroxide solution, the temperature is controlled at -10 to -5°C, after the dropwise addition, keep warm for 15 minutes, after the end, extract with 300L ethyl acetate, separate layers, remove the ethyl acetate layer, and adjust the pH to 1.2 with 2mol / L hydrochloric acid , and then extracted with 300L dichloromethane, the water layer was partitioned, the dichloromethane layer was dried with 20kg anhydrous calcium chloride, filtered, the filtrate was distilled under reduced pressure to obtain 62kg of oily matter, and N-phenylacetyl-L-proline was obtained after cooling acid, its purity is 99.5%.
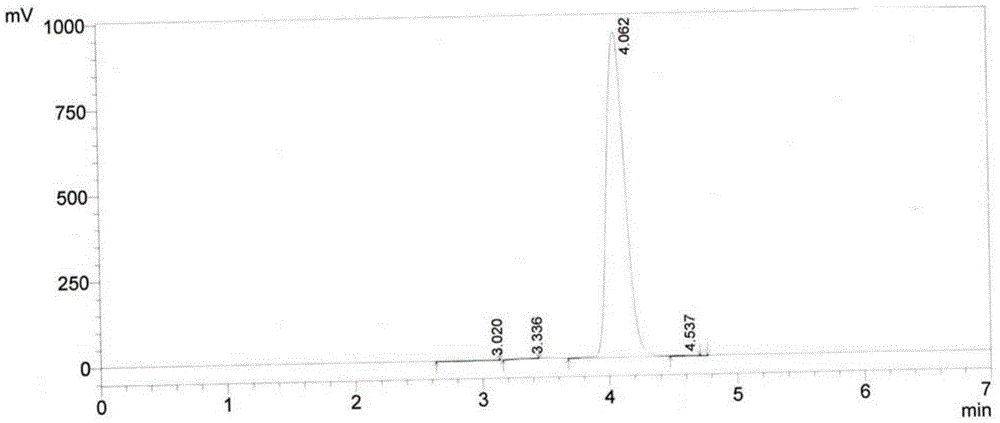
[0037] The N-phenylacetyl-L-proline prepared above is detected b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com