C17-heteroaryl derivatives of oleanolic acid and methods of use thereof
An alkyl, hydroxyl technology, used in biology and medicine, to solve problems such as differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
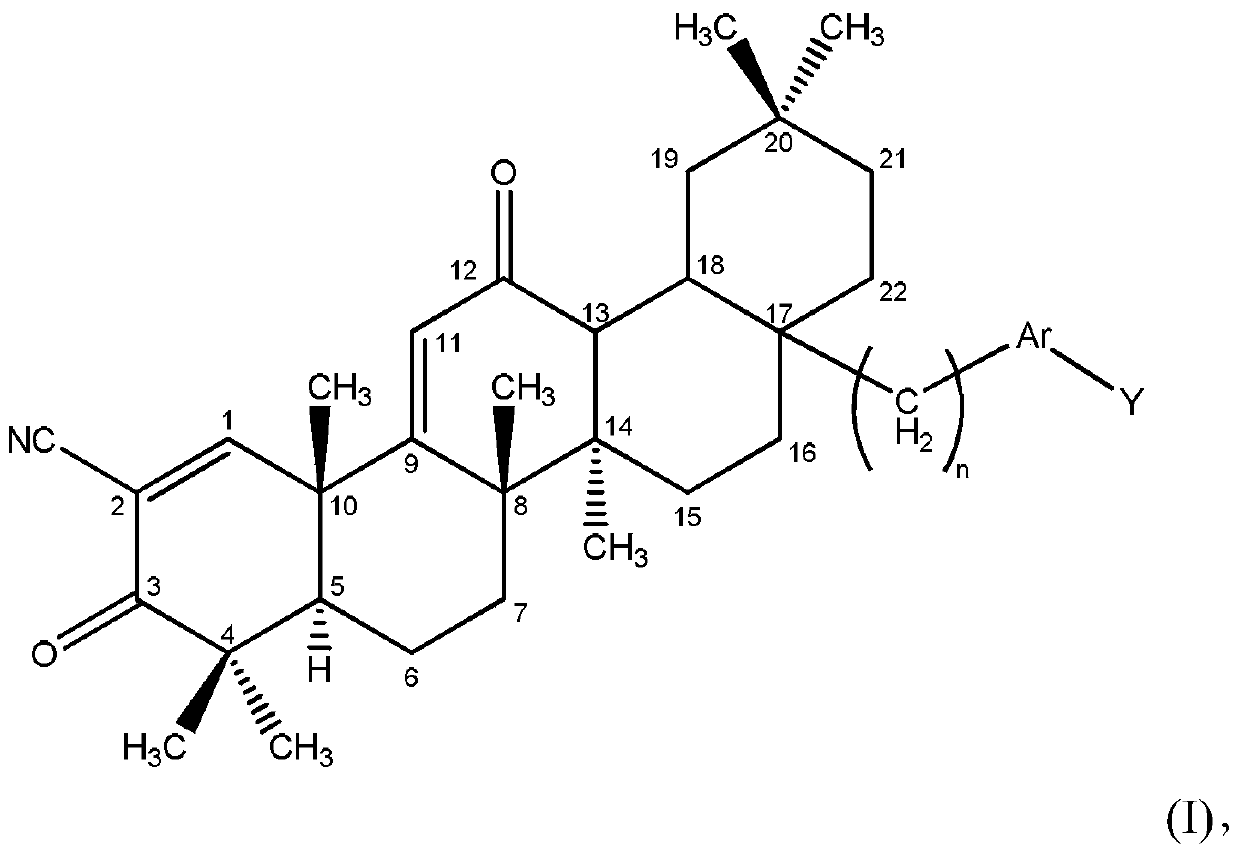
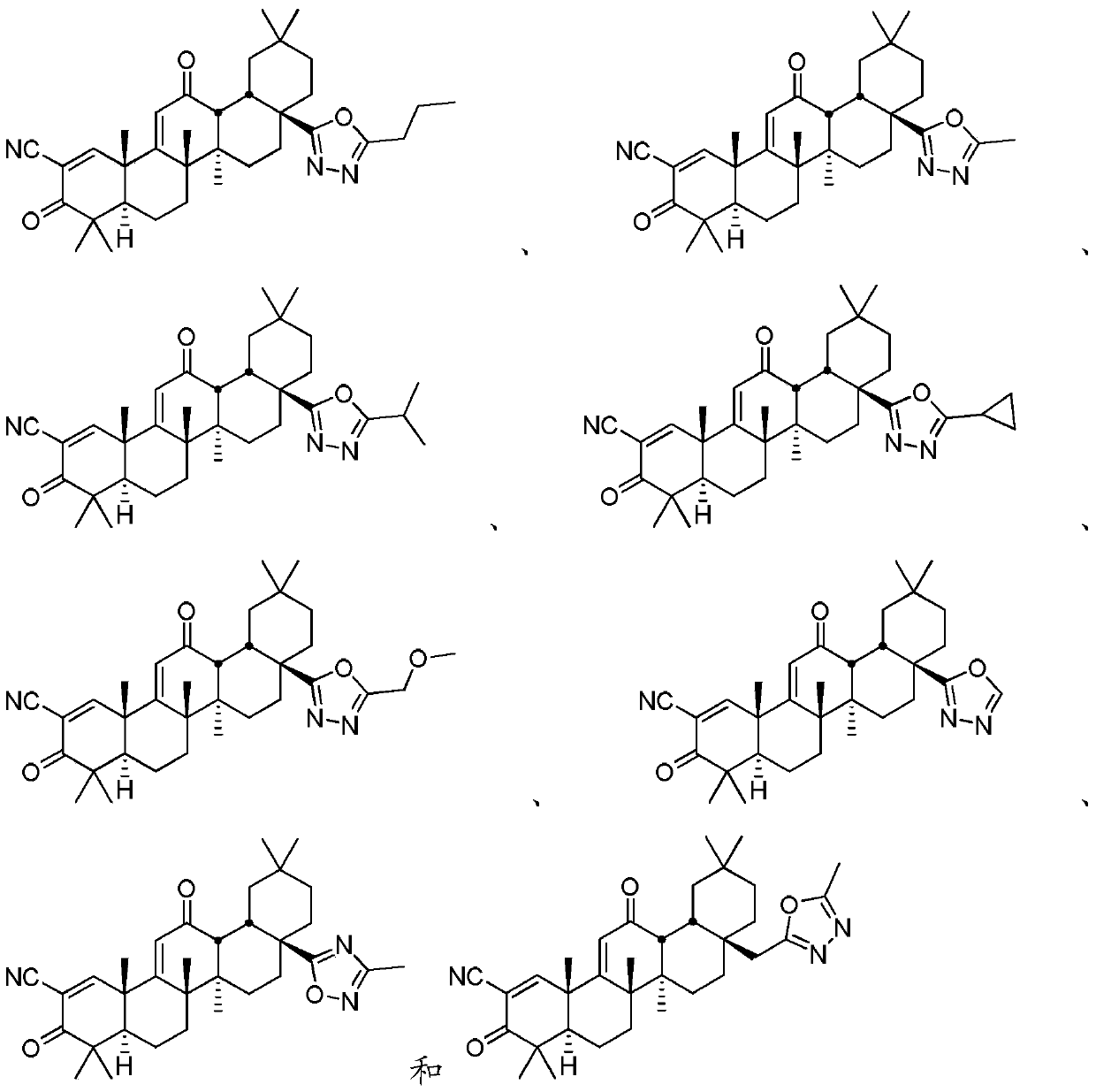
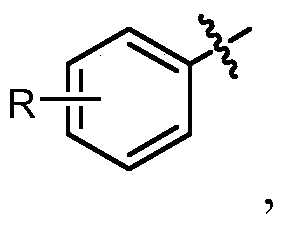
[0025] Disclosed herein are novel compounds and compositions having antioxidant and / or anti-inflammatory properties, methods of their manufacture, and methods of their use, including methods for treating and / or preventing disease.
[0026] I. Definition
[0027] When used in the context of chemical groups: "hydrogen" means -H; "hydroxyl" means -OH; "oxo" means =O; "carbonyl" means -C( =O)-; "Carboxyl" means -C(=O)OH (also written as -COOH or -CO 2 H); "halo" means independently -F, -Cl, -Br or -I; "amino" means -NH 2 ; "Hydroxyamino" means -NHOH; "nitro" means -NO 2 ; imino means =NH; "cyano" means -CN; "isocyanate" means -N=C=O; "azido" means -N 3 ; in a monovalent context, "phosphate group" means -OP(O)(OH) 2 or its deprotonated form; in a divalent context, "phosphate" means -OP(O)(OH)O- or its deprotonated form; "mercapto" means -SH; and "thio / Thio" means =S; "sulfonyl" means -S(O) 2 -; and "sulfinyl" means -S(O)-.
[0028] In the context of a chemical formula, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com