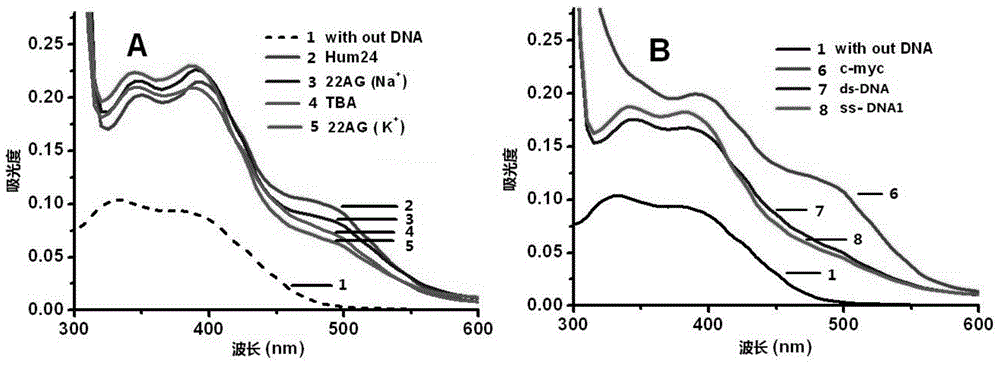
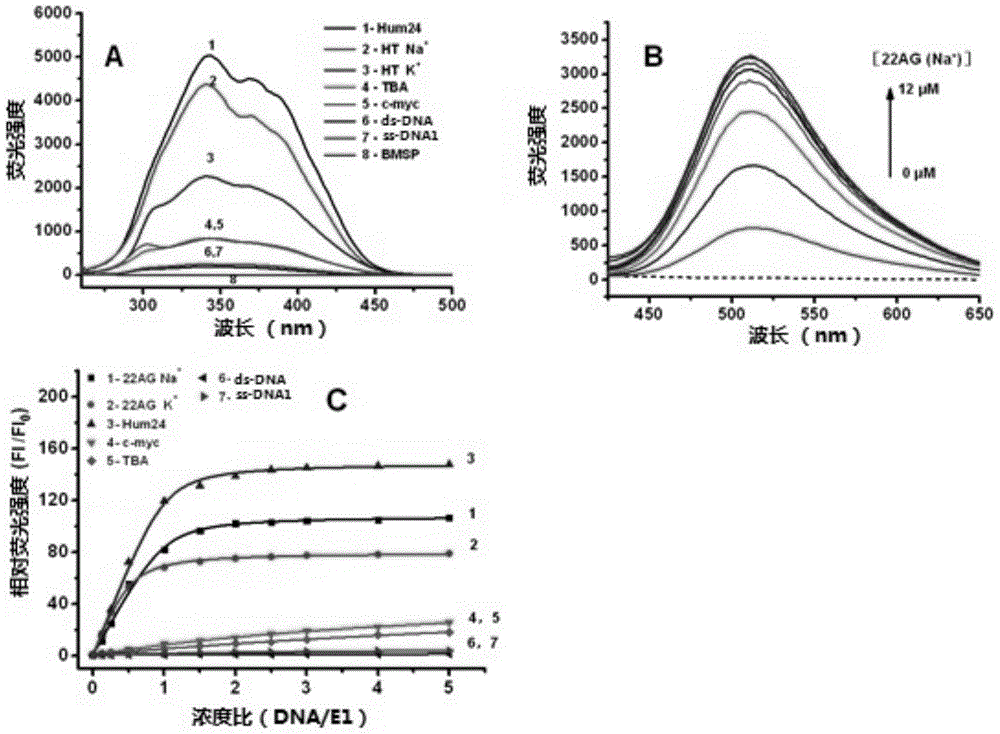
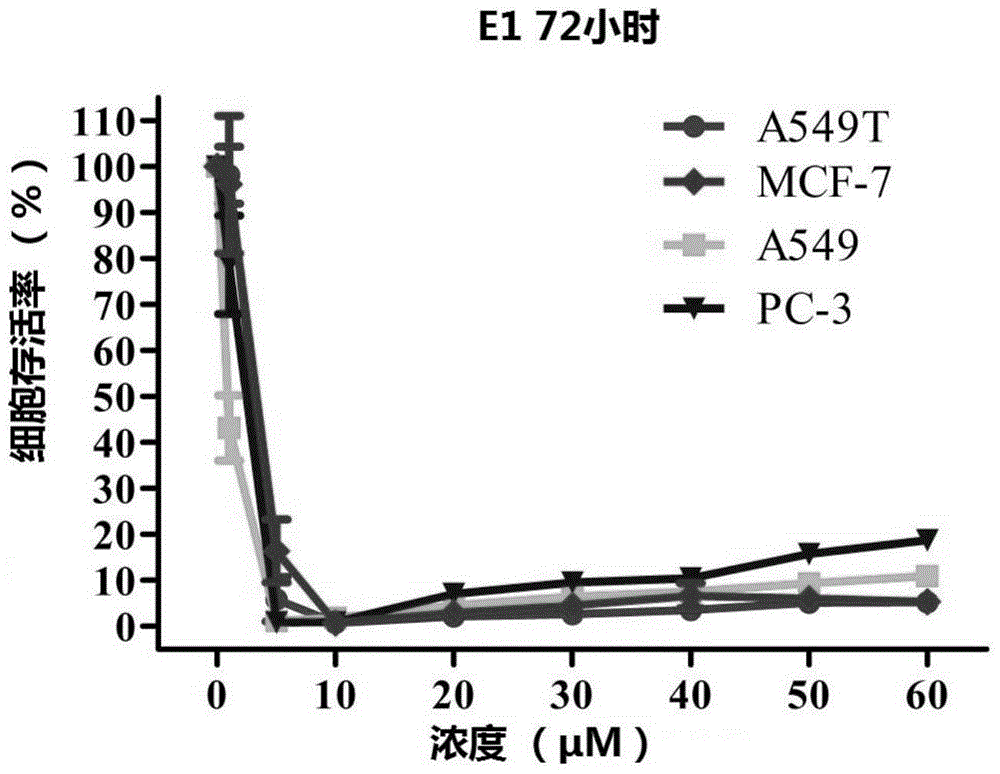
2,9-bisstyrene-substituted o-phenanthroline compounds, preparation method and application thereof
A compound and unsubstituted technology, applied in the field of medicine, can solve the problems of low G-quadruplex selectivity, insignificant changes in optical properties, and inability to detect with G-quadruplex structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1, the synthesis of compound E1
[0073] 4-(4-Methylpiperazine) benzaldehyde (purchased from Bailingwei) (2.04g, 10mmol) and 2,9-dimethyl-2,9-phenanthroline (purchased from Bailingwei) (1.04g, 5mmol) was dissolved in dry toluene (10mL), reacted at a temperature of 120°C for 24 hours, and the solvent in the system was spin-dried to obtain a crude product, which was purified by silica gel column chromatography with dichloromethane as an eluent to obtain 975mg of The solid is compound E1, and the yield is 35%.
[0074] The confirmed data of the compound structure are:
[0075] 1 H NMR (CDCl 3 ,400MHz):δ(ppm):8.04(d,2H),7.95(d,2H),7.83(m,4H),7.64(d,4H),7.42(d,2H),7.02(d,2H) ,6.85(m,2H),3.34(t,8H),2.85(d,8H),2.87(s,6H). 13 C NMR (CDCl 3 ,400MHz): δ(ppm): 156.71, 151.24, 145.84, 136.25, 133.84, 128.45, 127.85, 127.06, 125.50, 120.02, 115.51, 54.95, 48.32, 46.13. HRMS (ESI-TOF) calcd for C 38 h 41 N 6 [M] + 581.3387,found 581.3386.
Embodiment 2
[0076] Embodiment 2, the synthesis of compound E2
[0077] Basically identical with embodiment 1, difference is to replace 4-(4-methylpiperazine) benzaldehyde with compound 4-(4-(2-hydroxyethyl) piperazine) benzaldehyde (purchased from Bailingwei), Reaction at 120°C for 24 hours, rotary evaporation of toluene to obtain a crude product, which was separated by silica gel column chromatography using ethyl acetate as eluent to obtain compound E2. Yield: 38%.
[0078] The confirmed data of the compound structure are:
[0079] 1 H NMR (CDCl 3 ,400MHz):δ(ppm):8.04(d,2H),7.95(d,2H),7.83(m,4H),7.64(d,4H),7.42(d,2H),7.02(d,2H) ,6.85(m,2H),3.65(s,2H),3.34(m,20H),2.53(t,4H). 13 C NMR (CDCl 3 ,400MHz): δ(ppm): 156.71, 151.24, 145.84, 136.25, 133.84, 128.45, 127.85, 127.06, 125.50, 120.02, 115.51, 59.41, 56.32, 51.33. HRMS (ESI-TOF) calcd for C 40 h 44 N 6 o 2 [M] + 640.3526,found 640.3528.
Embodiment 3
[0080] Embodiment 3: the synthesis of compound E3
[0081] Basically the same as Example 1, the difference is to replace 4-(4-methylpiperazine) benzaldehyde with compound 4-(4-morpholine) benzaldehyde (purchased from Bailingwei), react at 120 ° C for 24 hours, and rotate Toluene was evaporated to obtain a crude product, which was subjected to silica gel column chromatography with ethyl acetate as eluent to obtain compound E3. Yield: 42%.
[0082] The confirmed data of the compound structure are:
[0083] 1H NMR (CDCl 3 ,400MHz):δ(ppm):8.04(d,2H),7.95(d,2H),7.83(m,4H),7.64(d,4H),7.42(d,2H),7.02(d,2H) ,6.85(m,2H),3.65(t,8H),3.18(t,8H). 13 C NMR (CDCl 3 ,400MHz): δ(ppm): 156.71, 151.24, 145.84, 136.25, 133.84, 128.45, 127.85, 127.06, 125.50, 120.02, 115.51, 66.35, 54.62. HRMS (ESI-TOF) calcd for C 36 h 34 N 4 o 2 [M] + 554.2682,found 554.2679.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com