Gefitinib glycol solvate and its preparation method and use
A technology of gefitinib glycol and solvate, which is applied in the field of gefitinib ethylene glycol solvate and its crystalline form to prepare high-purity gefitinib Form 1 crystal form, which can solve the problems of unfavorable industrial production, inability to It is better applicable to the problems of industrial production and high boiling point, and achieves the effects of easy industrial preparation, good controllability and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation of embodiment 1 gefitinib glycol solvate
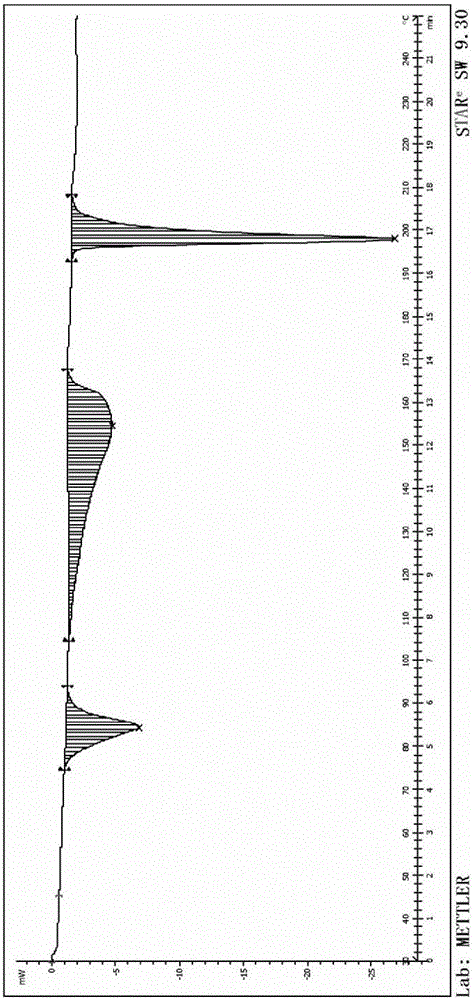
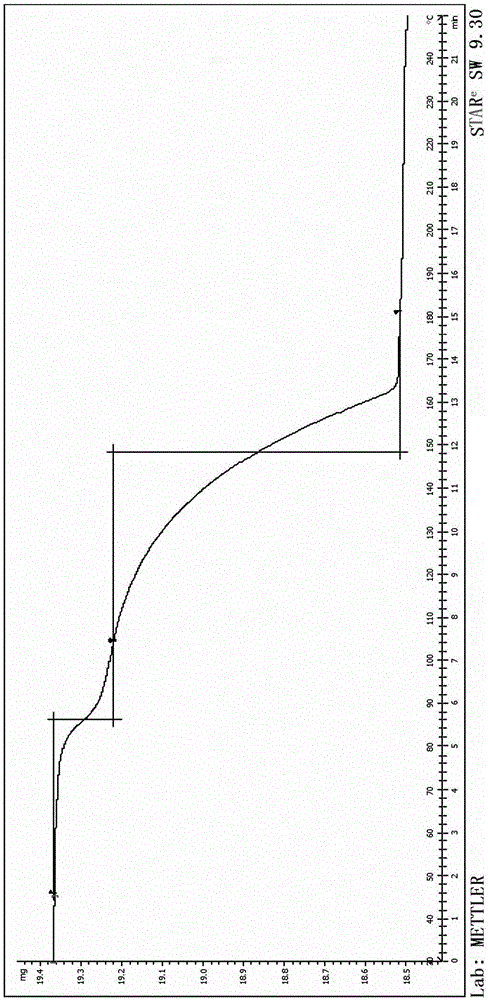
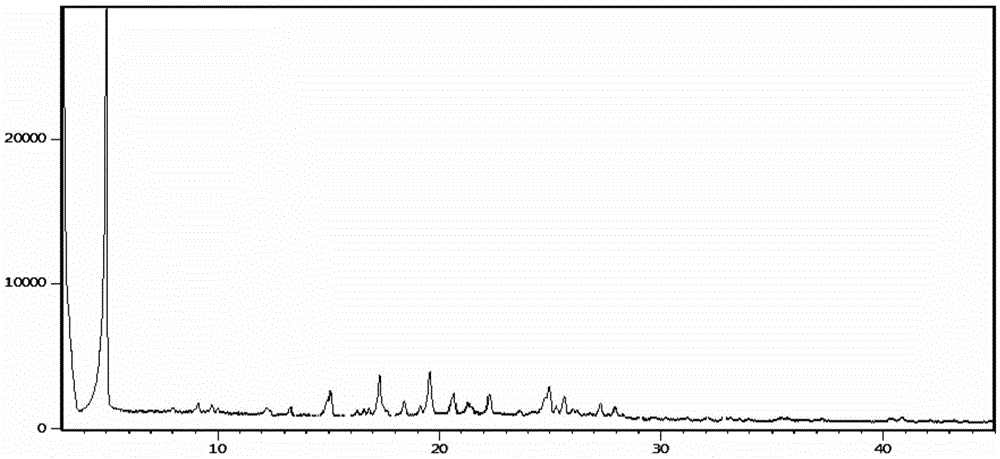
[0049] Add 40kg of 4-(3-chloro-4-fluoroaniline)-7-methoxyquinazolin-6-ol into 100kg of N,N-dimethylformamide, then add 40kg of potassium carbonate, N- (3-Chloropropyl)morpholine 25kg; react at 80-100°C for 4h, filter to remove insoluble inorganic salts; add ethylene glycol 1000kg to the filtrate, centrifuge and dry to obtain 60.9kg of gefitinib glycolate , yield 85.2%, purity 99.65%, ethylene glycol mass content 20.2%,; after determination, its X-RPD is as figure 1 As shown, its DSC spectrum is as figure 2 As shown, its TGA spectrum is as image 3 shown.
Embodiment 2
[0050] The preparation of embodiment 2 gefitinib glycol solvates
[0051] Add 40g of 4-(3-chloro-4-fluoroaniline)-7-methoxyquinazolin-6-ol to 100g of N,N-dimethylformamide, then add 40g of potassium carbonate, N- (3-Chloropropyl)morpholine 25g; react at 80-90°C for 6h, filter to remove insoluble inorganic salts; add ethylene glycol 300g to the filtrate, filter with suction, and dry to obtain gefitinib glycolate 56.9 g, yield 79.6%, purity 99.55%, ethylene glycol content 21.1%; after determination, its X-RPD collection of patterns and figure 1 Basically the same, its DSC spectrum and figure 2 Basically the same.
Embodiment 3
[0052] The preparation of embodiment 3 gefitinib glycol solvates
[0053] Add 40g of 4-(3-chloro-4-fluoroaniline)-7-methoxyquinazolin-6-ol to 100g of N,N-dimethylformamide, then add 40g of potassium carbonate, N- (3-Chloropropyl)morpholine 25g; react at 70-80°C for 8h, filter to remove insoluble inorganic salts; add ethylene glycol 2000g to the filtrate, filter, and dry to obtain 61.4g of gefitinib glycolate , yield 85.9%, purity 99.57%, ethylene glycol content 21.3%; after determination, its X-RPD pattern and figure 1 Basically the same, its DSC spectrum and figure 2 Basically the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com