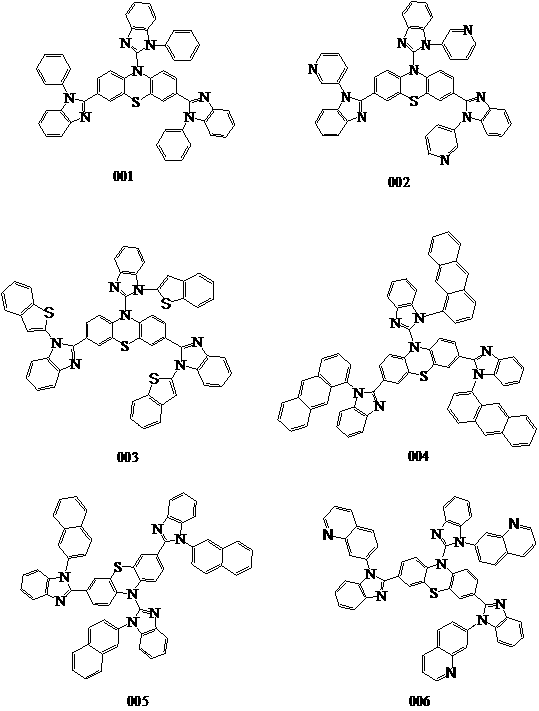
Green material, preparation method and application thereof
A reaction and derivative technology, applied in the field of organic electroluminescent materials, can solve the problems of not meeting the requirements of OLED use, and achieve the effect of low manufacturing cost and reduced manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The present invention also provides a preparation method of benzimidazole phenothiazine derivatives, wherein the reaction scheme is shown in the following formula, and the specific steps are:
[0050]
[0051] S100, 2-bromo-1H-benzimidazole and R1-substituted iodine substitution reaction to obtain R1-substituted benzimidazole compounds;
[0052] S200, reacting 10H-phenothiazine-3,7-diboronic acid and a benzimidazole compound substituted by R1 group to obtain a benzimidazole phenothiazine diboronic acid derivative containing R1 substituent;
[0053]S300, reacting the benzimidazole compound substituted with R1 group and the benzimidazole phenothiazine diboronic acid derivative containing R1 substituent to obtain the benzimidazole phenothiazine derivative containing R1 substituent;
[0054] The preparation method of the phenothiazine derivatives, wherein the S100 specifically includes the following steps:
[0055] 2-Bromo-1H-benzimidazole and R1-substituted iodine are ...
Embodiment 1
[0074]
[0075] Weigh 120.00mmol of p-iodotoluene, 80.00mmol of 2-bromo-1H-benzo[d]imidazole, 120.00mmol of potassium tert-butoxide, 4.00mmol of palladium (II) acetate, and 4.00mmol of tri-tert-butylphosphine, and dissolve them in 250ml of toluene , under the protection of nitrogen, reacted at 80°C for 5 hours, filtered the reaction solution, and recrystallized with petroleum ether and dichloromethane to generate 66.64mmol of 2-bromo-1-(p-methyl)-1H-benzo[d]imidazole , the yield is above 83.3%, and the HPLC purity is greater than 98%.
[0076] Weigh 20.00mmol of 10H-phenothiazine-3,7-diboronic acid, 40.00mmol of 2-bromo-1-(p-methyl)-1H-benzo[d]imidazole, 40.00mmol of potassium tert-butoxide, palladium acetate ( Ⅱ) 1.40mmol, 1.40mmol of tri-tert-butylphosphine, dissolved in 120ml of toluene, reacted at 80°C for 5 hours under the protection of nitrogen, filtered the reaction solution, recrystallized with petroleum ether and dichloromethane to obtain the intermediate, and cont...
Embodiment 2
[0078]
[0079] 122mmol of 3-iodopyridine and 80mmol of 2-bromo-1H-benzo[d]imidazole, 130mmol of potassium tert-butoxide, 4.20mmol of palladium (II) acetate, 4.20mmol of tri-tert-butylphosphine, dissolved in 250ml of toluene, under nitrogen protection 2-bromo-1-(p-methyl)-1H-benzo[d]imidazole 67.60mmol, yield 84.5 % or more, HPLC purity greater than 98%.
[0080] Weigh 20.00mmol of 10H-phenothiazine-3,7-diboronic acid, 42.00mmol of 2-bromo-1-(3-pyridyl)-1H-benzo[d]imidazole, 42.00mmol of potassium tert-butoxide, palladium acetate (II) 1.60mmol, 1.50mmol of tri-tert-butylphosphine, dissolved in 120ml of toluene, reacted at 80°C for 5 hours under the protection of nitrogen, filtered the reaction solution, recrystallized with petroleum ether and dichloromethane to obtain the intermediate, continued to add 40mmol of 2-bromo-1-(3-pyridyl)-1H-benzo[d]imidazole, 40mmol of potassium tert-butoxide, 1.40mmol of palladium (II) acetate, 1.40mmol of tri-tert-butylphosphine, dissolve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com