A treatment solution and a method for determining antigen content in aluminum salt-adsorbed vaccines using it
A technology of antigen content and treatment solution, applied in the biological field, can solve the problems of in vitro detection of antigen content and quality control of antigen content, etc., and achieve the effect of effective detection, high accuracy and strong durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
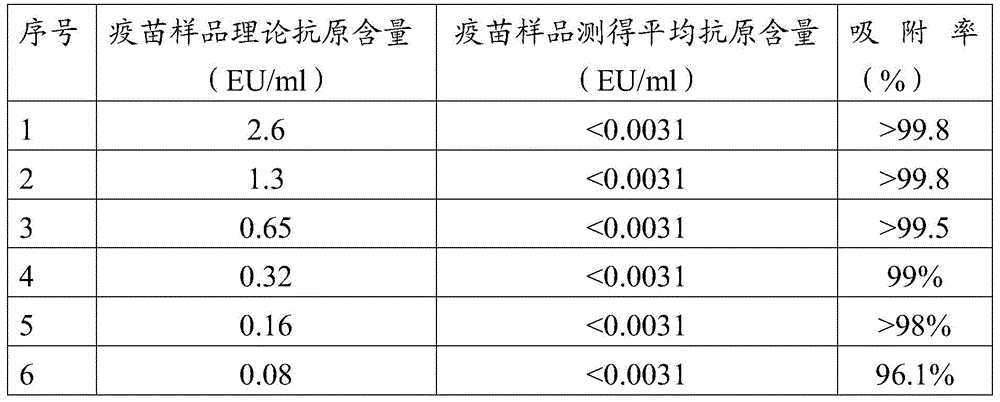
[0050] Example 1 Detect the antigen content of the sample processing solution, and detect the antigen content of six different concentrations of adsorbed Japanese encephalitis inactivated vaccine samples.
[0051] Take 0.702ml, 0.252ml, 0.176ml, 0.088ml, 0.044ml, and 0.022ml of the vaccine stock solution with an antigen concentration of 7.4EU / ml and add them to the test tubes, and add aluminum hydroxide adjuvant solution ( Beijing Bofengke Biotechnology Co., Ltd. ) to a final concentration of aluminum hydroxide solution / mixture = 20% (v / v), and add ultrapure water to make up the volume of each tube to 2ml. That is to say, vaccine samples with a theoretical antigen content of 0.08EU / ml, 0.16EU / ml, 0.32EU / ml, 0.65EU / ml, 1.3EU / ml, and 2.6EU / ml were prepared, and four vaccines were prepared for each concentration. These vaccine samples containing different antigen concentrations were detected according to the following steps:
[0052] (1) Detection of adsorption rate
[0053] ...
Embodiment 2
[0083] Example 2 Influence of bovine serum albumin content in sample treatment solution on the detection of antigen content in adsorbed Japanese encephalitis inactivated vaccine samples
[0084] On the basis of the sample treatment solution in (2-1) in Example 1, respectively prepare bovine serum albumin containing 5% (w / v), 10% (w / v), 15% (w / v), 20% (w / v), 30% (w / v), and 40% (w / v) sample treatment solutions were used to investigate their impact on the detection method. details as follows:
[0085] According to the method in Example 1, six samples of adsorbed Japanese encephalitis inactivated vaccine containing 1.3EU / ml JE antigen were prepared, and 5%, 10%, 15%, 20%, 30%, The sample treatment solution of 40% bovine serum albumin was processed according to the treatment method in Example 1, and then the antigen content was detected, and the result calculation method was also as described in Example 1. The implementation results are shown in Table 5.
[0086] Table 5 Examp...
Embodiment 3
[0089] Example 3 Describe the preparation ratio of the sample treatment solution and the adsorbed inactivated Japanese encephalitis vaccine sample.
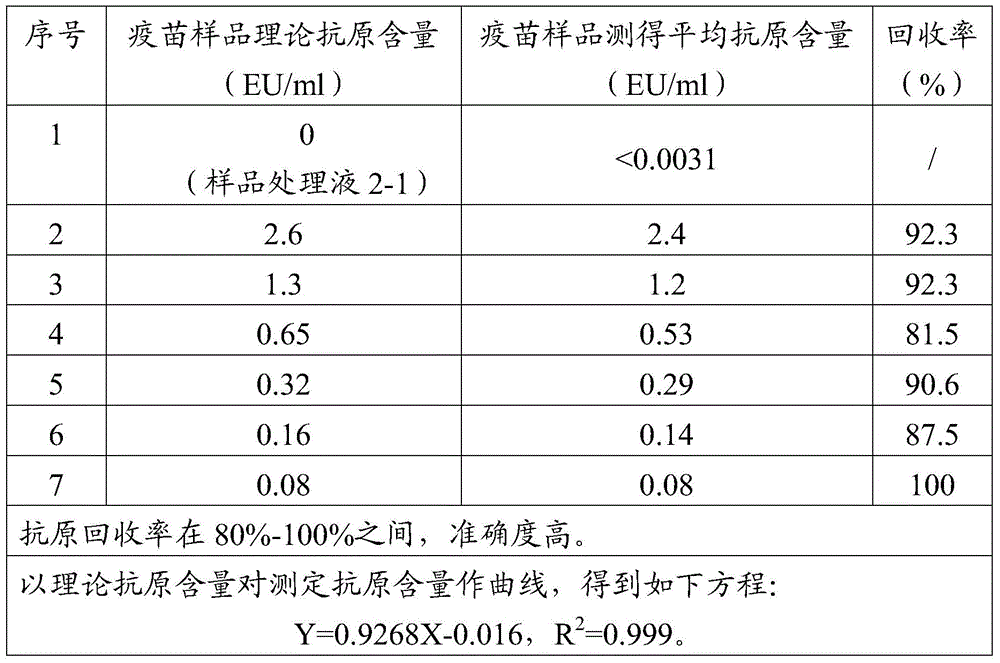
[0090] According to the method in Example 1, six sticks of adsorbed Japanese encephalitis inactivated vaccine samples 1ml containing 1.3EU / ml JE antigen were prepared, and 10ml, 5ml, 1ml, 0.5ml, 0.1ml, 0.05ml of (2-1) Sample treatment solution, other steps were treated according to the treatment method in Example 1, and then the antigen content was detected, and the result calculation method was also as described in Example 1. The implementation results are shown in Table 6.
[0091] Table 6 Example 3 result summary
[0092]
[0093] Analysis: As can be seen from the results in Example 3, after the vaccine to be tested and the sample treatment solution are prepared according to the volume ratio of 1:10 to 1:0.1, the final antigen recovery rate is similar, both being 92.3%. When the volume of the sample treatment solution r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com