Methods for passivating metallic implantable medical devices including radiopaque markers
A medical device and marker technology, applied in the field of manufacturing implantable medical devices, can solve the problems of no available space, increasing the wall thickness of the stent, affecting the flexibility of the stent, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
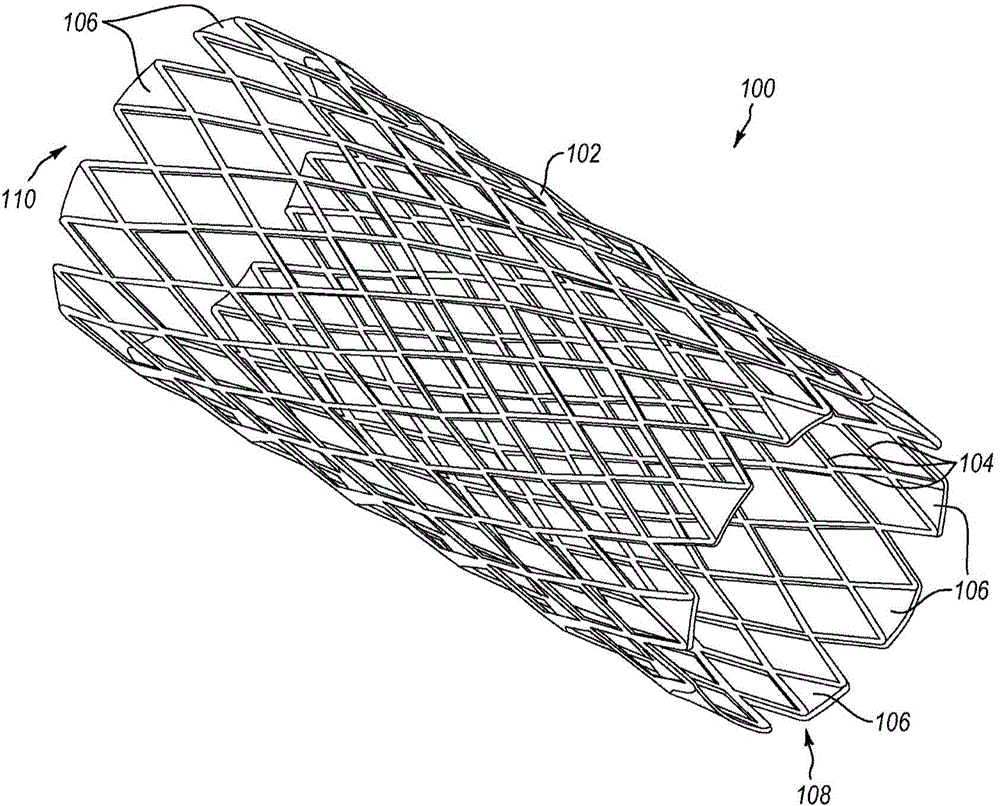
[0038] In one embodiment, the present method can achieve passivation of the stent body as well as radiopaque markers by using electropolishing rather than traditional chemical passivation techniques. For example, while electropolishing does function to remove metallic material from the electropolished structure, it also results in the formation of a thin passivating film layer (eg, an oxide of the underlying metal) on the surface of the structure. According to one embodiment, electropolishing is achieved by immersing the structure to be electropolished in an electrolyte solution and subjecting the immersed structure to an applied electrical current, causing anodic metal dissolution of the metal on the surface of the structure.
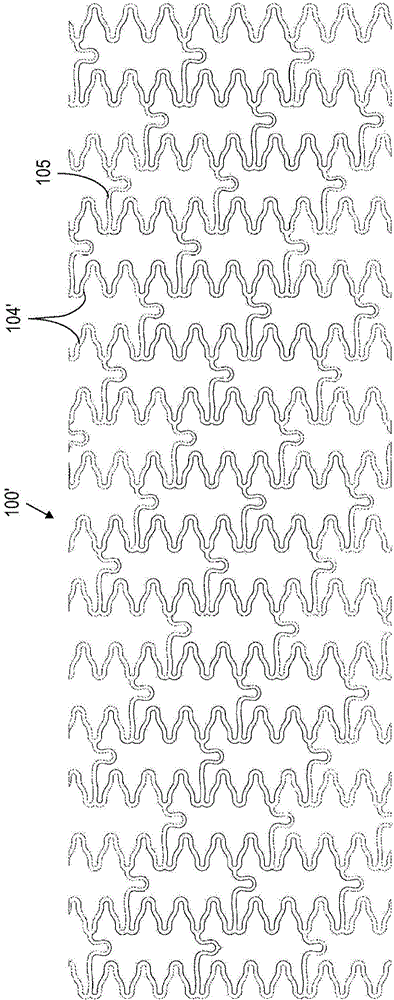
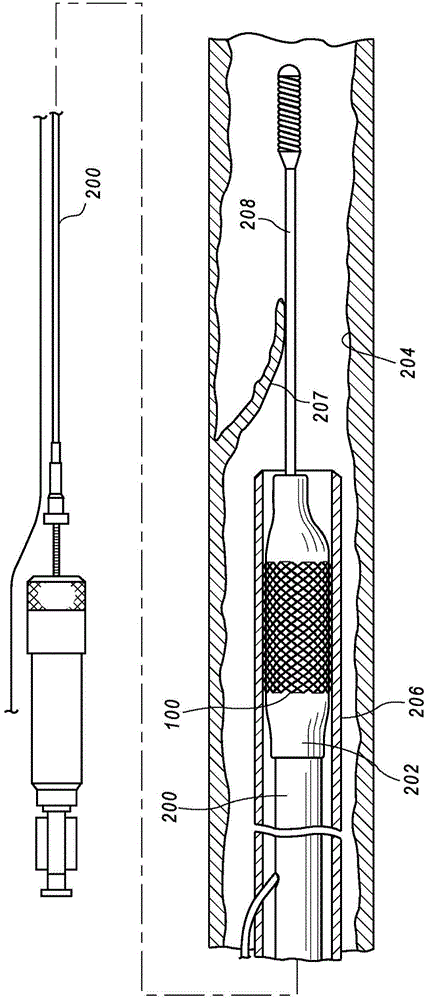
[0039] Such as image 3 As shown, according to method S10, an implantable medical device (eg, a tubular stent body) is provided at S12. Prior to attaching the radiopaque markers, a preliminary electropolishing (S14) of the device body smoothes the sur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| surface roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com