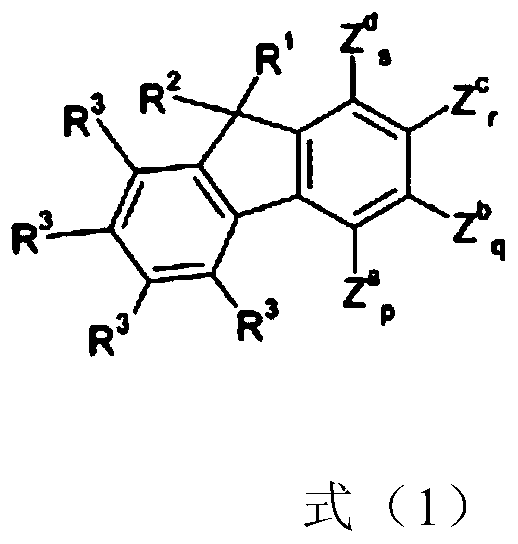
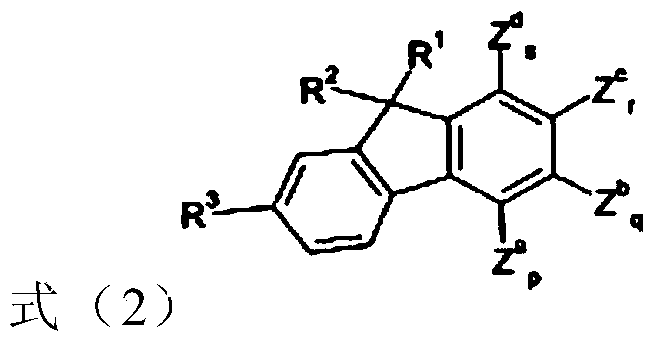
Compounds and Organic Electroluminescent Devices
A technology for electroluminescent devices and compounds, applied in the field of preparing said compounds and organic electroluminescent devices, capable of solving problems such as poor performance data and high operating voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0307] Synthesis of compound biphenyl-2-yl-biphenyl-4-yl-(9-methyl-9-p-tolyl-9H-fluoren-2-yl)-amine (1-1) and compound (1-2) To (1-11)
[0308]
[0309] Among them, Toluene is toluene, and Buchwald-Hartwig coupling is Buchwald-Hartwig coupling.
[0310] 2-bromo-9-methyl-9-p-tolyl-9H-fluorene
[0311] 40 g (154 mmol) of 2-bromo-9H-fluorene was dissolved in 500 mL of dry THF in a thoroughly heated flask. Solution with N 2 Saturate and add 15.0 g (170 mmol) of cerium(III) chloride. The clear solution was cooled to -10°C and then 121 mL (170 mmol) of 1.4M methylmagnesium bromide solution was added. The reaction mixture was slowly warmed to room temperature, and then NH 4 Cl quench (500 mL). The mixture was then partitioned between acetate and water, the organic phase was washed three times with water and passed through Na 2 SO 4 Dry and rotary evaporate. Add 60 mL of toluene to the rotary evaporated solution. The precipitate was heated to 50°C and then 27.2 mL of trifluoromethanesu...
example 2
[0323] Synthetic compound biphenyl-4-yl-(9,9-dimethyl-9H-fluoren-2-yl)-(9-methyl-9-phenyl-9H-fluoren-4-yl)-amine (2 -1) and compounds (2-2) to (2-8)
[0324]
[0325] Among them, Aminierung means amination
[0326] 4-bromo-9-methyl-9-phenyl-9H-fluorene
[0327] 30 g (94 mmol) of 2,2'-dibromobiphenyl was dissolved in 200 mL of dry THF in a thoroughly heated flask. The reaction mixture was cooled to -78°C. At this temperature, 37.7 mL of a 2.5M solution of n-butyl lithium in hexane (94 mmol) was slowly added dropwise (duration: about 1 hour). The precipitate was stirred at -70°C for 1 hour. Subsequently, 11.1 mL of acetophenone (94 mmol) was dissolved in 100 ml of THF and added dropwise at -70°C. After the addition was complete, the reaction mixture was slowly warmed to room temperature, with NH 4 The Cl is quenched and then concentrated on a rotary evaporator. Carefully add 300 mL of acetic acid to the rotary evaporated solution, and then add 50 mL of fuming hydrochloric acid. ...
example 3
[0337] Synthetic compound biphenyl-4-yl-biphenyl-2-yl-(7,9-diphenyl-9-p-tolyl-9H-fluoren-2-yl)-amine (3-1) and compound (3 -2) to (3-4)
[0338]
[0339] Among them, Aminierung means amination, Kupplung means coupling, and Toluen means toluene.
[0340] 2-bromo-7-phenylfluorene-9-one
[0341] 21.6g (178mmol) of phenylboronic acid and 60g (178mmol) of 2,7-dibromo-fluorene were suspended in 800mL of dimethoxyethane and 265mL (533mmol) of 2M sodium carbonate solution. 6.154 g (5 mmol) of tetrakis(triphenylphosphine) was added to the suspension, and the reaction mixture was heated to reflux for 18 hours. After the reaction mixture was cooled, the organic phase was separated, filtered on silica gel, washed 3 times with 100 mL of water and then concentrated to dryness. After filtering the crude product through toluene on silica gel, 38.6 g (85%) of 2-bromo-7-phenylfluoren-9-one was obtained.
[0342] The following brominated compounds were prepared similarly:
[0343]
[0344] 2-bromo-7,9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com