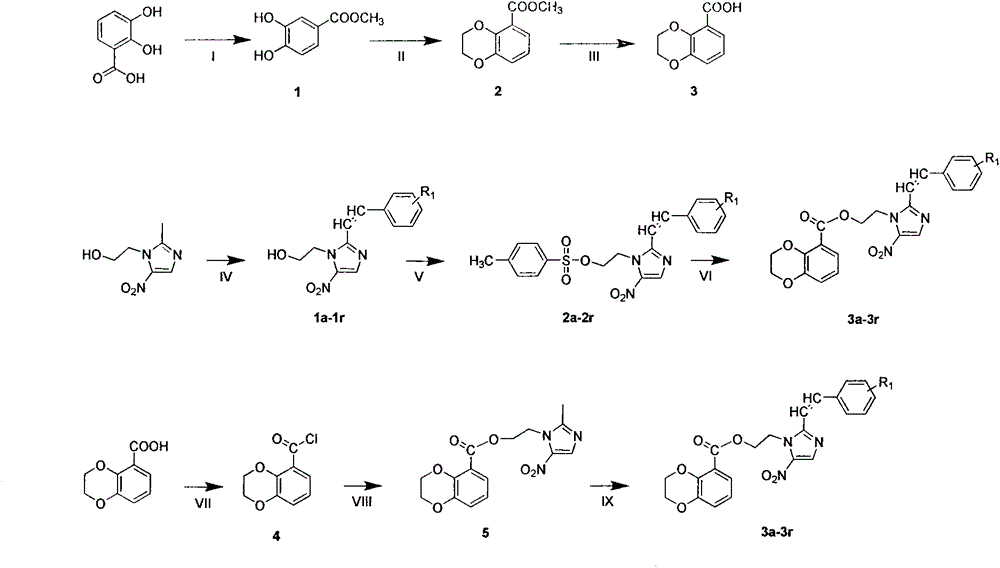
Synthesis and bio-activity evaluation of 2-styryl-5-nitroimidazol derivatives containing 1,4-benzdioxan skeleton
A technology of benzodioxane and nitroimidazole is applied in the field of synthesis of novel 2-styryl-5-nitroimidazole derivatives, and can solve the problems of obvious adverse reactions, poor pharmacokinetic properties and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
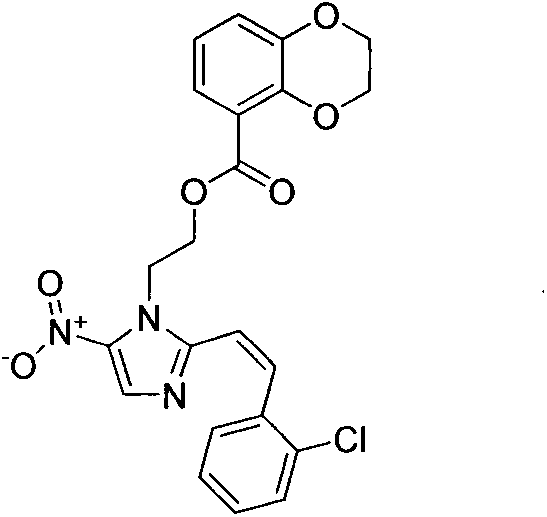
[0015] Example 1: (Z)-2-(2-(2-chlorostyryl)-5-nitroimidazole)ethyl 2,3-dihydrobenzo[b][1,4]dioxa Preparation of cyclohexene-5-carboxylic acid (3a)
[0016]
[0017] After dissolving catechin (1 mmol) in methanol (30 ml), concentrated sulfuric acid (0.5 ml) was added dropwise and kept overnight at 90°C. After the reaction, the solvent was removed, the organic layer was dissolved with ethyl acetate (20ml), and then extracted with water (40ml), the organic layer was dried with anhydrous sodium sulfate, the solvent was removed under reduced pressure, and a solid was precipitated. The solid was recrystallized from ethanol to obtain compound 1, filled with nitrogen, dissolved compound 1 (1mmol) in anhydrous acetone (10ml), then added anhydrous potassium carbonate (2mmol), and added dropwise a mixed solution of dibromomethane (1mmol) and acetone , reflux reaction for 24 hours. After the reaction solution was distilled off under reduced pressure, an appropriate amount of water wa...
Embodiment 2
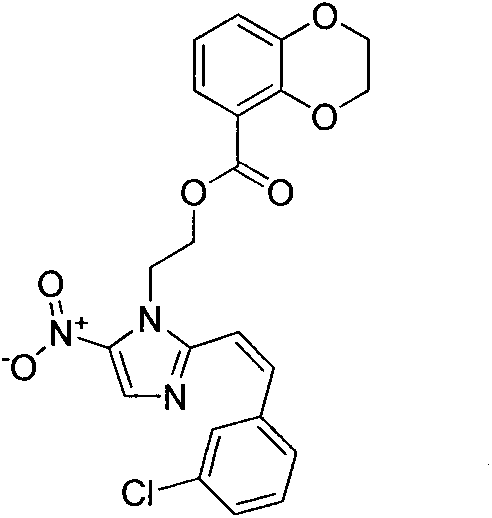
[0021] Example 2: (Z)-2-(2-(3-chlorostyryl)-5-nitroimidazole)ethyl 2,3-dihydrobenzo[b][1,4]dioxa Preparation of cyclohexene-5-carboxylic acid (3b)
[0022]
[0023] The preparation method is the same as that in Example 1, and the target compound 3b is obtained by replacing o-chlorobenzaldehyde with m-chlorobenzaldehyde. Yield 64.5%.m.p.188~189℃; 1 H NMR (DMSO-d 6 , 300MHz) δ: 8.25(s, 1H), 8.01(t, J=7.1Hz, 2H), 7.77-7.69(m, 2H), 7.42(t, J=7.5Hz, 1H), 7.17(t, J =7.6Hz, 1H), 6.93(d, J=7.8Hz, 1H), 6.42(d, J=7.9Hz, 2H), 5.06(t, J=4.4Hz, 2H), 4.75(t, J=4.2 Hz, 2H), 4.17(s, 4H).
Embodiment 3
[0024] Example 3: (Z)-2-(2-(4-chlorostyryl)-5-nitroimidazole)ethyl 2,3-dihydrobenzo[b][1,4]dioxa Preparation of cyclohexene-5-carboxylic acid (3c)
[0025]
[0026] The preparation method is the same as in Example 1, and p-chlorobenzaldehyde is used instead of o-chlorobenzaldehyde to obtain the target compound 3c. Yield 67.5%.m.p.187~189℃; 1 H NMR (DMSO-d 6 , 300MHz) δ: 8.25(s, 1H), 7.89(s, 1H), 7.71(d, J=12.3Hz, 1H), 7.64-7.61(m, 1H), 7.52(d, J=7.6Hz, 1H ), 7.42(d, J=6.8Hz, 2H), 7.11(d, J=7.9Hz, 1H), 6.91(d, J=8.1Hz, 1H), 6.53-6.48(m, 1H), 5.02(t , J=8.4Hz, 2H), 4.57(t, J=8.2Hz, 2H), 4.13(s, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com