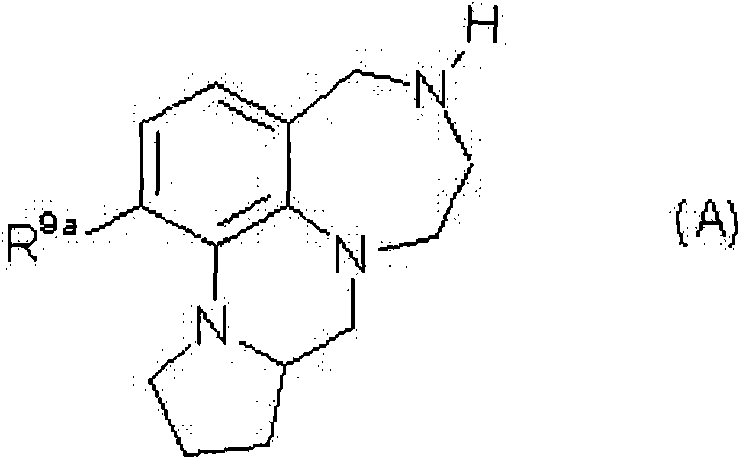
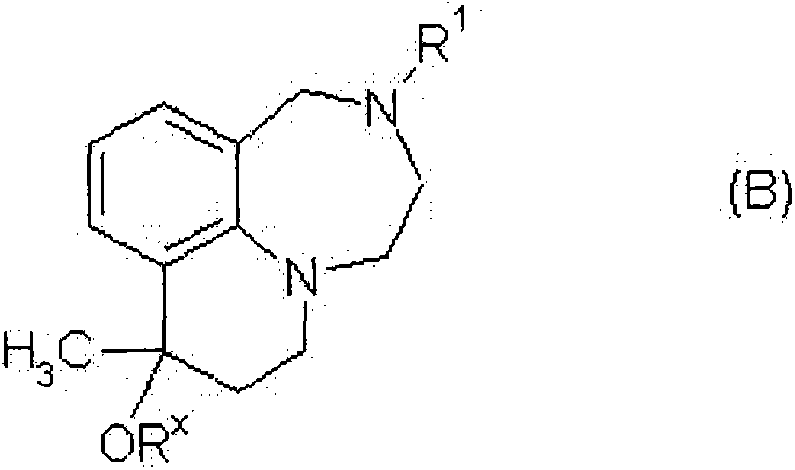
Tricyclic chinoline and quinoxaline derivatives
A technology of quinoxalines and compounds, which is applied in drug combinations, digestive system, metabolic diseases, etc., and can solve problems affecting patient compliance and limiting treatment efficacy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
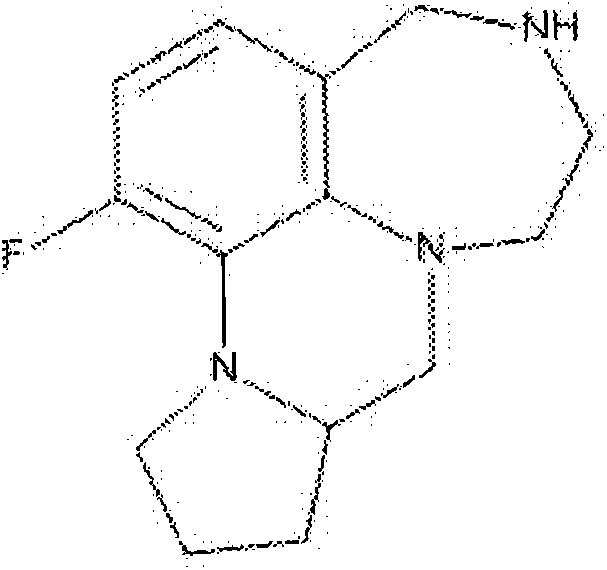
[0101] 1-fluoro-5,6,7,9,9a,10,11,12-octahydro-4H-[1,4]diazepine And[1,7,6-de]pyrrolo[1,2-a]quinoxaline, trifluoroacetic acid
[0102]
[0103] 1.1 Preparation of pyrrolidine-2-methyl carboxylate
[0104] SOCl at 0°C 2 (250ml) was added dropwise to a solution of pyrrolidine-2-carboxylic acid (75g, 651mmol) in methanol (750ml) and the reaction mixture was stirred at 20°C for 16 hours. Set up an additional vial in the same manner. The two reaction mixtures were combined and concentrated under reduced pressure to obtain the title compound (90 g, 543 mmol, yield 50%).
[0105] 1 H NMR (400MHz, DMSO-d 6 ): δ[ppm]: 9.83(s, 1H), 4.32(t, J=7.6Hz, 1H), 3.72(s, 3H), 3.21-3.15(m, 2H), 2.22-2.20(m, 1H) , 1.96-1.87(m, 3H)
[0106] 1.2 Preparation of 1-(2-fluoro-6-nitro-phenyl)-pyrrolidine-2-carboxylic acid methyl ester
[0107] Triethylamine (26.3 mL, 189 mmol) and 1,2-difluoro-3-nitrobenzene (15 g, 94 mmol) were added to methyl pyrrolidine-2-carboxylate (17.18 g, 104 mmol) in a...
Embodiment 2
[0133] 1-bromo-5,6,7,9,9a,10,11,12-octahydro-4H-[1,4]diazepine And[1,7,6-de]pyrrolo[1,2-a]quinoxaline
[0134]
[0135] 2.1 Preparation of 11-(2-bromo-6-nitro-phenyl)-pyrrolidine-2-carboxylate
[0136] A mixture of 1-bromo-2-fluoro-3-nitrobenzene (60 g, 273 mmol), methyl pyrrolidine-2-carboxylate (54.2 g, 327 mmol; see Example 1.1) and triethylamine (83 g, 818 mmol) Heated at 70°C for 16h. The mixture was then cooled, diluted with ethyl acetate (1000ml), washed successively with 2N HCl (500ml), K 2 CO 3 Aqueous solution (300ml) and brine (300ml) were washed and the aqueous phase was re-extracted with ethyl acetate (500ml). Ethyl acetate layer with Na 2 SO 4 Drying, filtration, concentration, and the residue was purified by column chromatography on silica gel (eluted with petroleum ether: ethyl acetate = 50:1 to 10:1) to give the title compound as a yellow solid (66 g, yield rate of 74%).
[0137] 1 HNMR (400MHz, CDCl 3 ), δ [ppm] 7.80-7.78 (m, 1H), 7.64-7.62 (m, ...
Embodiment 3
[0155] 1-methyl-5,6,7,9,9a,10,11,12-octahydro-4H-[1,4]diazepine And[1,7,6-de]pyrrolo[1,2-a]quinoxaline, trifluoroacetic acid
[0156]
[0157] 3.1Boc-protected 1-bromo-5,6,7,9,9a,10,11,12-octahydro-4H-[1,4]diazepine Preparation of [1,7,6-de]pyrrolo[1,2-a]quinoxaline
[0158] Triethylamine (6.4g, 63.3mmol) and Boc 2 A solution of O (10.1 g, 46.4 mmol) in DCM (20 ml) was added to 1-bromo-5,6,7,9,9a,10,11,12-octahydro-4H-[1,4]diazepine miscellaneous A solution of a[1,7,6-de]pyrrolo[1,2-a]quinoxaline (13 g, 42.2 mmol) in dichloromethane (DCM) (260 ml) was then allowed to warm to 23 ° C and stirred for 16h. The reaction was diluted with DCM (250ml), washed with 2N HCl (200ml), saturated K 2 CO 3 Aqueous solution (150ml) and brine (150ml) were washed, and the aqueous phase was re-extracted with DCM (200ml). The combined organic phases were washed with Na 2 SO 4 Dry, filter, and concentrate, and the residue was purified by column chromatography on silica gel (eluted w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com