Method for obtaining soluble proinsulin by utilizing Escherichia coli expression system
An expression system and Escherichia coli technology, applied in the directions of insulin, botanical equipment and methods, biochemical equipment and methods, etc., can solve the problems of complicated operation and high cost, and achieve the effect of reducing production cost and improving expression and yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
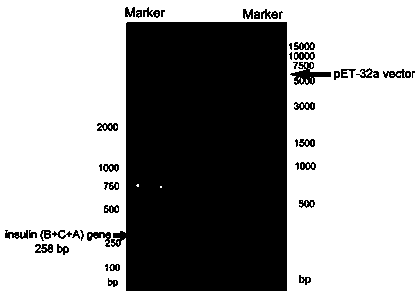
[0051] Construction of TrxA-PI fusion expression vector
[0052] (1) According to the published human-derived preproinsulin gene sequence (Genbank CAA23828.1) in the NCBI database, the codon was optimized as E. coliPreferring codons, the full gene sequence of PI was synthesized at BGI:
[0053] 1 ATG GCA CTG TGG ATG CGT CTG CTG CCG CTG CTG GCC CTG CTG GCC 45
[0054] 1 Met Ala Leu Trp Met Arg Leu Leu Pro Leu Leu Ala Leu Leu Ala 15
[0055] 46 CTG TGG GGT CCG GAT CCA GCC GCA GCA TTC GTT AAT CAG CAT CTG 90
[0056] 16 Leu Trp Gly Pro Asp Pro Ala Ala Ala Phe Val Asn Gln His Leu 30
[0057] 91 TGT GGC AGC CAT CTG GTG GAA GCG CTG TAT CTG GTT TGC GGT GAA 135
[0058] 31 Cys Gly Ser His Leu Val Glu Ala Leu Tyr Leu Val Cys Gly Glu 45
[0059] 136 CGT GGC TTT TTC TAT ACC CCG AAA ACC CGT CGT GAA GCG GAA GAT 180
[0060] 46 Arg Gly Phe Phe Tyr Thr Pro Lys Thr Arg Arg Glu Ala Glu Asp 60
[0061] 181 CTG CAG GTG GGC CAG GTG GAA CTG GGT GGC GGT CCA GGC GCA GGC 225
[0062] 61 Leu...
Embodiment 2
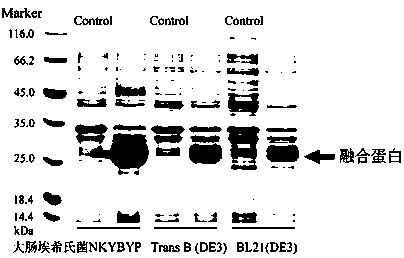
[0076] Transform different expression strains and compare the expression of fusion proteins
[0077] The plasmids verified by sequencing were transferred into three expression strains: E. coli BL21 (DE3) (Novagen), E. coli transB (DE3) (Beijing Quanshijin Company) and Escherichia coli NKYBYP. Single clones on the plate were picked and cultured overnight in LB medium. E. coli Add Amp at a final concentration of 100 μg / ml to BL21 (DE3) medium; E. coli Amp, 15 μg / ml Kan, and 12.5 μg / ml Tet were added to transB (DE3) medium at a final concentration of 100 μg / ml; Amp, 100 μg / ml final concentration was added to Escherichia coli NKYBYP medium 15 μg / ml Kan, 12.5 μg / ml Tet, 34 μg / ml Chl (the same below).
[0078] The cultured bacterial solution was inoculated into 30 ml culture medium according to the inoculum amount of 1%, and expanded at 37°C and 200 rpm. When OD 600 When = 0.8, a final concentration of 1 mM IPTG was added, induced at 37°C and 160 rpm for 8 h, and the...
Embodiment 3
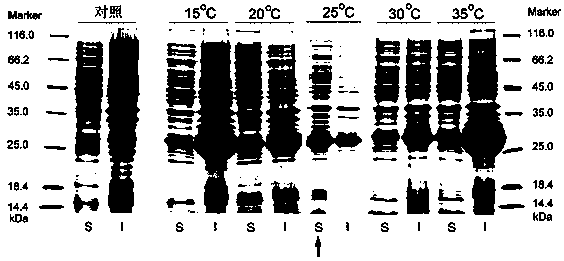
[0083] Optimization of induction conditions
[0084] (1) Induction temperature
[0085] Since the TrxA-PI fusion protein has the highest expression level in Escherichia coli NKYBYP, only the induction conditions of this strain were optimized to increase the expression level of the soluble fusion protein.
[0086] The cultured bacterial solution was inoculated into 30 ml culture medium according to the inoculum amount of 1%, and expanded at 37°C and 200 rpm. When OD 600 = 0.8, the final concentration of 1 mM IPTG was added, and the bacterial solution was induced at 15°C, 20°C, 25°C, 30°C, 35°C, 160 rpm for 8 h.
[0087] Collect the cells by centrifugation at 11000 rpm for 2 minutes, wash the cells twice with triple-distilled water, add 5 ml of 1×binding buffer (0.5 M NaCl, 20 mM Tris-HCl, 5 mM imidazole, pH 8.0) to dissolve the cells, and sonicate , Sonication conditions: power 350 watts, ultrasonic for 5 seconds, interval of 10 seconds, ultrasonic 30 times until the bacte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com