Preparation method of bromo-substituted benzimidazole derivative
A technology of benzimidazole and derivatives, applied in the field of novel preparation of pharmaceutical intermediates, can solve problems such as difficulty in synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
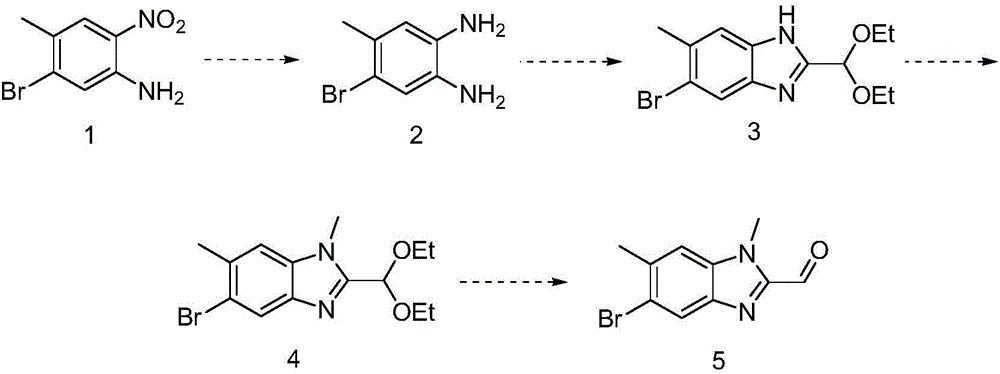
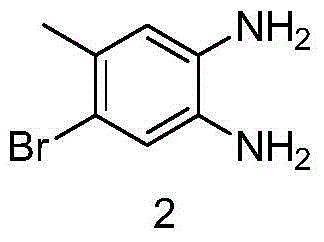
[0022] (1) Synthesis of 4-bromo-5-methyl-1,2-phenylenediamine
[0023] Add 36g of 5-bromo-4-methyl-2-nitroaniline to 400ml of ethanol, add 3g of 10% palladium carbon, feed hydrogen, stir overnight, filter, collect the filtrate, concentrate to obtain 27g of 4-bromo-5 -Methyl-1,2-phenylenediamine.
[0024] (2) Synthesis of 5-bromo-2-(diethoxymethyl)-6-methyl-1H-benzo[d]imidazole
[0025] Add 28g of 4-bromo-5-methyl-1,2-phenylenediamine to 350ml of absolute ethanol, add 17g of sodium ethoxide, then add 32g of ethyl 2,2-diethoxyacetate, heat and reflux for 24 hours , cooled, concentrated, added water and ethyl acetate for extraction and separation, collected the organic phase, dried, and concentrated to obtain 19g of 5-bromo-2-(diethoxymethyl)-6-methyl-1H-benzo[ d] imidazole.
[0026] (3) Synthesis of 5-bromo-2-(diethoxymethyl)-1,6-dimethyl-1H-benzo[d]imidazole
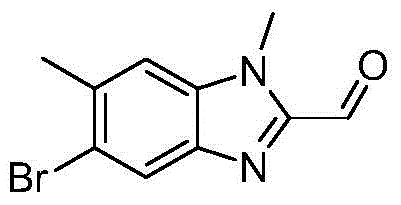
[0027] 19g of 5-bromo-2-(diethoxymethyl)-6-methyl-1H-benzo[d]imidazole was added to 150ml of N,N-dimethylformamide,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com