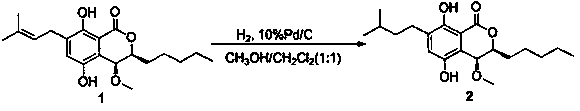Marine fungi secondary metabolite derivative and application of marine fungi secondary metabolite derivative as marine biological antifoulant
A technology of marine organisms and antifouling agents, applied in biocides, plant growth regulators, chemicals for biological control, etc., can solve problems such as damage to the marine environment, poisoning, and immune system damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] Weigh out 10 mg compound 1 Dissolve in 5mL CH 3 OH / CH 2 Cl 2 (volume ratio 1:1), adding a catalytic amount of 10% Pd / C, at 1 atm H 2 After 10 h of reaction at room temperature, the reaction was complete by TLC. After filtration to remove Pd / C, after concentration, 9.9 mg of oil was obtained, with a yield of 98.4%. The purity by HPLC was above 96%, and ESI-MS m / z: 373.2 [M+Na] + .
Embodiment 2
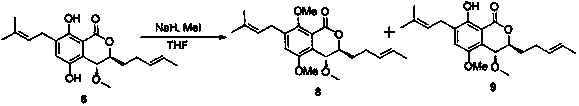
[0032]
[0033] Weigh out 20 mg compound 3 Dissolve in 5 mL CH 2 Cl 2 , adding a catalytic amount of PtO 2 , at 1 atm H 2 Under the action, after reacting at room temperature for 4 hours, TLC detected that the reaction raw materials almost completely disappeared and formed two spots with low polarity, which were filtered to remove PtO 2 , after concentration, the compound was obtained by silica gel column chromatography (eluent: EtOAc / petroleum ether=10:1 ~ 8:1) 4 (13.5 mg), yield 67.2%, compound 5 (4.8 mg), yield 23.9%, HPLC purity above 98%, ESI-MS m / z: 371.2 [M+Na] + ,through 1 Comparison of H NMR spectra to identify compounds 4 , 5 structure, compound 4 There are no two Hs around δ5.4 (double-bonded hydrogen at the 5' and 6' positions), but there are two single peaks and three Hs around 1.7 ((C H 3 ) 2 C=C-), compound 5 On the contrary, there are two H around δ5.4 (double-bonded hydrogen at the 5' and 6' positions), two CH 3 ((C H 3 ) 2 CH-) moves upfie...
Embodiment 3
[0035]
[0036] Weigh out 10 mg compound 6 Dissolve in 5 mL THF, add 5 mg Na 2 CO 3 , after stirring at room temperature for half an hour, add 5 μL EtBr, 30 o After reacting at C for 5 h, TLC detected that the reaction raw materials almost completely disappeared. After concentration, the compound was obtained by silica gel column chromatography (eluent: EtOAc / petroleum ether=15:1 ~ 10:1). 7 (9 mg), the yield was 83.3%, the purity of the compound by HPLC was 98.6%, ESI-MS m / z: 397.2 [M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com