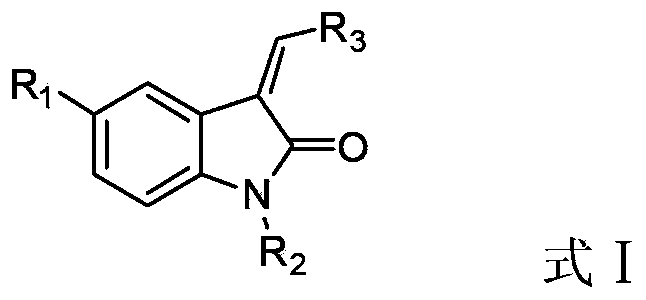
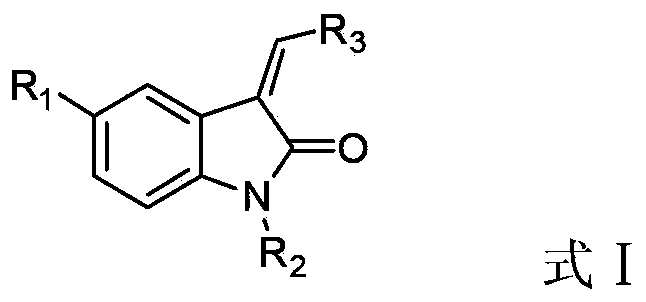
2-Indolone derivative with tyrosine kinase inhibition activity, and preparation method and application thereof
A tyrosine kinase and inhibitory activity technology, which is applied in the directions of organic active ingredients, medical preparations containing active ingredients, organic chemistry, etc., can solve problems such as the need to improve the efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Example 1, 3-[3-(2-dimethylaminoethoxy)-4-methoxybenzylidene]-5-[N-methyl-1-(3-hydroxyl-4-fluoro- Synthesis of anilinosulfo]-2-indolone hydrochloride (T1)
[0135]Add 2 mL of absolute ethanol to 0.1 g (0.3 mmol) of compound M3, add 0.08 g (0.36 mmol, 1.2 eq) of compound M4 under stirring, add two drops of piperidine, reflux in an oil bath for 30 min, and TLC detects that the reaction is complete. After standing overnight, a yellow solid precipitated out, which was filtered by suction, washed with a large amount of absolute ethanol, and detected by TLC as a single point. Dry to obtain 67.8mg, yield 42.4%. The solid was just dissolved in absolute ethanol, and HCl-ether solution was added dropwise until the pH was 2. After standing still, a yellow solid precipitated out. Suction filtration, washing with a large amount of ether until the filtrate is neutral. The yellow solid was dried to yield 52.5 mg. 1H-NMR (400Hz, DMSO-d6) δ (ppm): 2.20 (s, 3H), 2.61 (t, 2H), 3.00 (s...
Embodiment 2
[0137] Example 2, 3-{3-[2-(pyrrol-1-yl)ethoxy]-4-methoxybenzylidene}-5-[N-methyl-1-(3-hydroxyl- Synthesis of 4-fluoro-anilinosulfo]-2-indolone hydrochloride (T2)
[0138] Add 2 mL of absolute ethanol to 0.13 g (0.4 mmol) of compound M3, add 0.12 g (0.48 mmol, 1.2 eq) of compound M5 with stirring, add two drops of piperidine, reflux in an oil bath for 30 min, and TLC detects that the reaction is complete. Separation by column chromatography and gradient elution of dichloromethane-methanol gave 0.14 g of a yellow oil with a yield of 62.5%. The oily substance was just dissolved in absolute ethanol, and HCl-ether solution was added dropwise until the pH was 2. After standing still, a yellow solid precipitated out. Suction filtration, washing with a large amount of ether until the filtrate is neutral. The yellow solid was dried to yield 55.5 mg. 1H-NMR (400Hz, DMSO-d6) δ (ppm): 1.69 (m, 4H), 2.55 (m, 4H), 2.87 (t, 2H), 3.10 (s, 3H), 3.86 (s, 3H), 4.15(t,2H),6.41-6.49(m,1H),6.70...
Embodiment 3
[0140] Example 3, 3-{3-[2-(piperidin-1-yl)ethoxy]-4-methoxybenzylidene}-5-[N-methyl-1-(3-hydroxyl -Synthesis of 4-fluoro-anilinosulfo]-2-indolone hydrochloride (T3)
[0141] Add 2 mL of absolute ethanol to 0.13 g (0.4 mmol) of compound M3, add 0.12 g (0.48 mmol, 1.2 eq) of compound M6 while stirring, add two drops of piperidine, reflux in an oil bath for 30 min, and TLC detects that the reaction is complete. Separation by column chromatography and gradient elution of dichloromethane-methanol gave 0.21 g of a yellow oil with a yield of 91.3%. The oily substance was just dissolved in absolute ethanol, and HCl-ether solution was added dropwise until the pH was 2. After standing still, a yellow solid precipitated out. Suction filtration, washing with a large amount of ether until the filtrate is neutral. The yellow solid was dried to give 24.2 mg. 1H-NMR (400Hz, DMSO-d6) δ (ppm): 1.36-1.51 (m, 6H), 2.43-2.46 (m, 4H), 2.64 (t, 2H), 3.01 (s, 3H), 3.85 (s ,3H),4.05(t,2H),6.42-6.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com