Method for predicting effect of hydroxyl-group-substituted polybrominated diphenyl ethers on thyroid hormone and model establishing method
A technology of thyroid hormones and diphenyl ethers, which is applied in the field of rapid prediction of the effect of hydroxy-substituted polybrominated diphenyl ethers on thyroid hormones, can solve the problems of unsatisfactory prediction performance, large number of descriptors, and low transparency of the model, achieving Calculation and modeling methods are simple and easy to implement, with excellent predictive performance and labor-saving effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
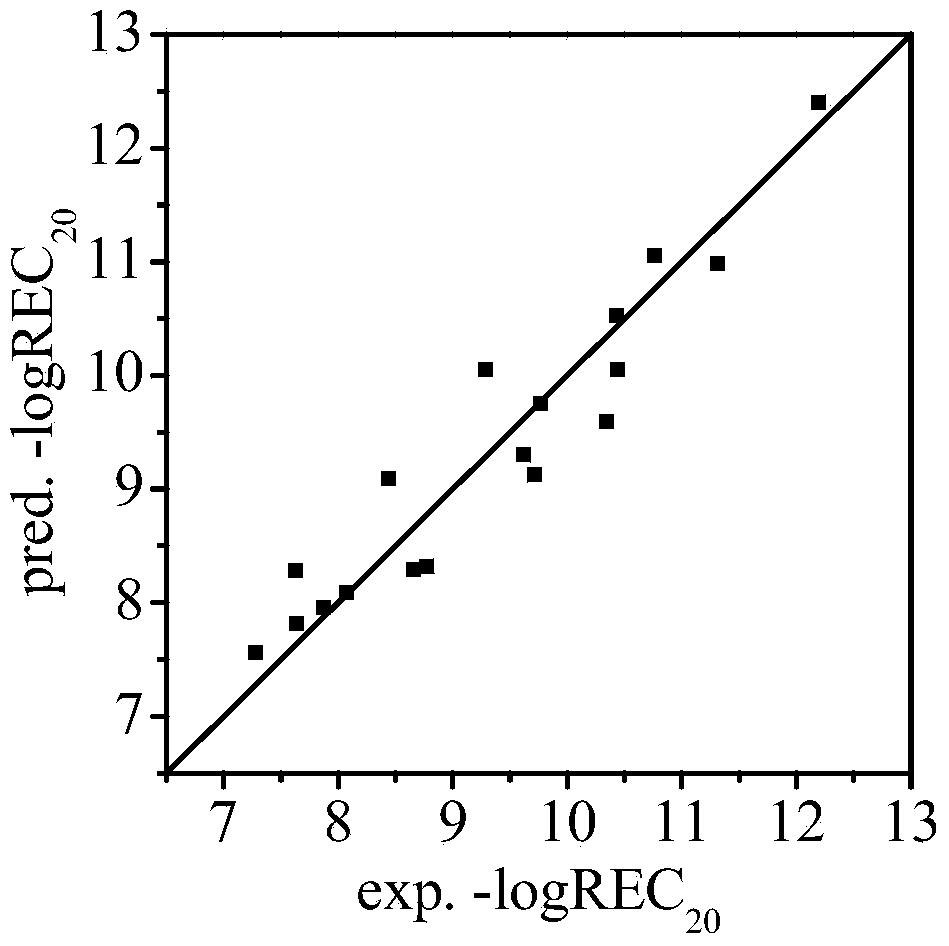
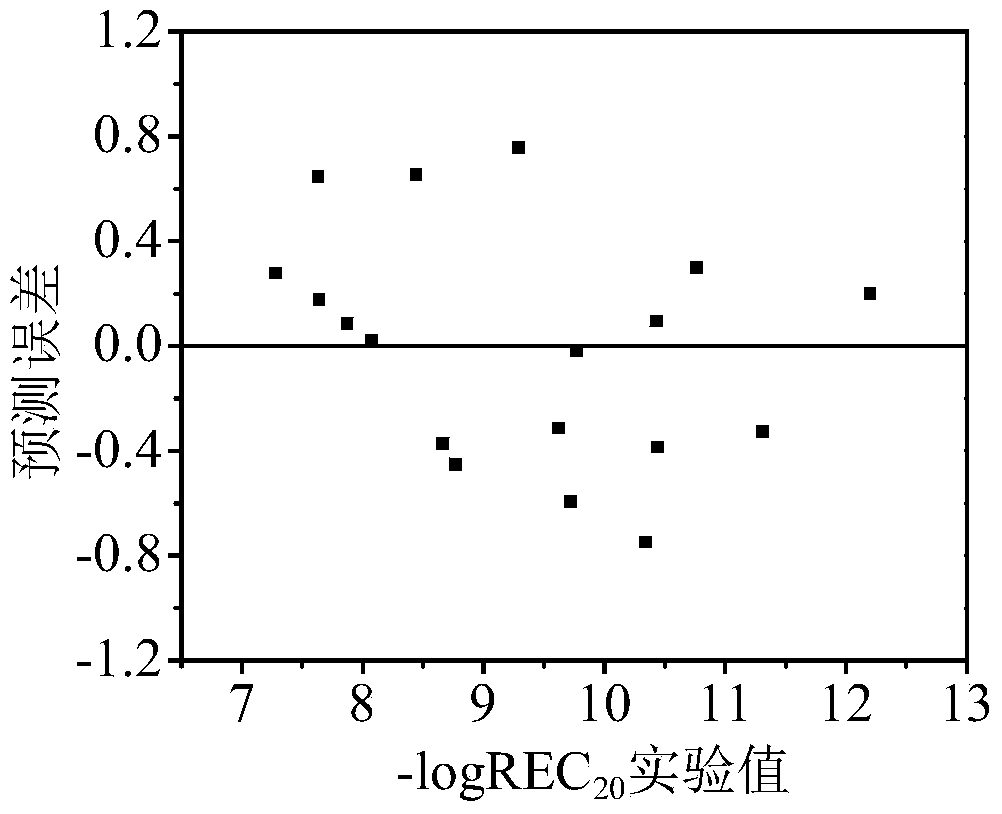
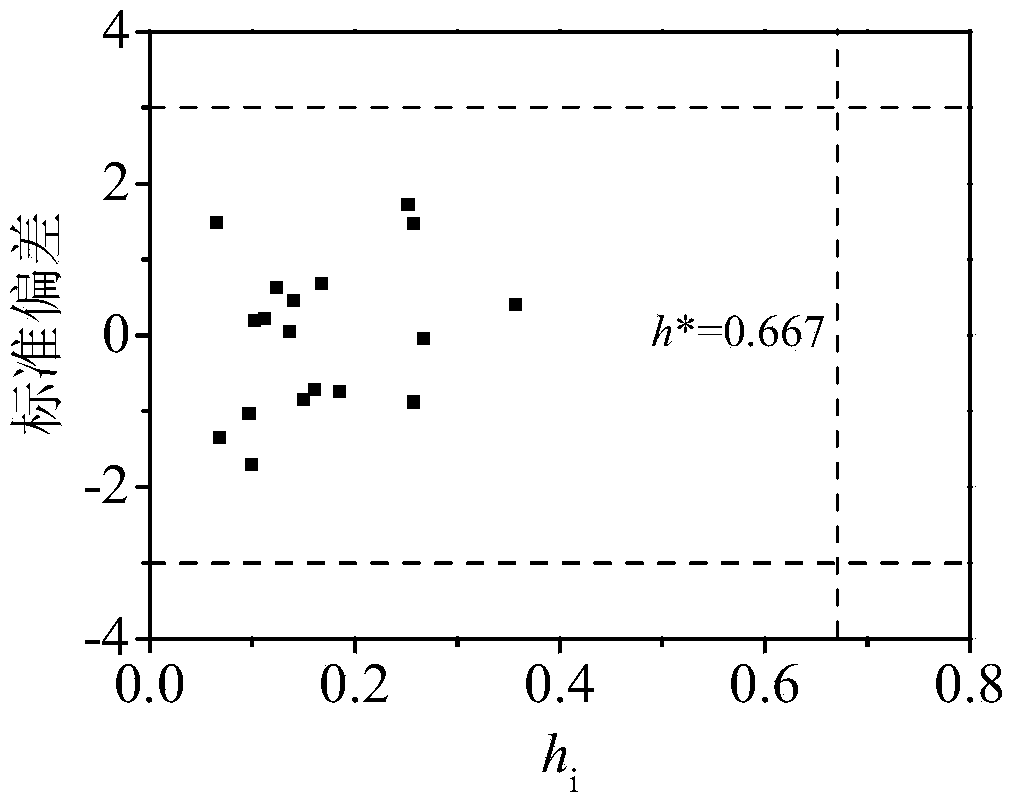
[0029] 2'-OH-BDE-28: Its h is calculated by Williams graph method i The value is 0.137<h*(warning value)=0.667, standard residual (SE)=0.046<3, indicating that this compound is within the application domain of the QSAR model. Three descriptors were calculated by using the HF / 6-31G** algorithm of Gaussian 03 and EPI Suite respectively.
[0030] --logREC of 2'-OH-BDE-28 20 The experimentally determined value was 8.07. The prediction steps based on the QSAR model are as follows:
[0031] -logREC 20 =24.22×QE occ (-8.8eV,O)+2.61×QE vac (3.8eV,H)+0.79×logK OW -15.96
[0032] =24.22×(0.425)+2.61×(3.633)+0.79×(5.40)-15.96
[0033] =8.09
Embodiment 2
[0035] 6-OH-BDE-85: Its h is calculated by Williams graph method i The value is 0.267-3, indicating that this compound is within the application domain of the QSAR model. Three descriptors were calculated by using the HF / 6-31G** algorithm of Gaussian 03 and EPI Suite respectively.
[0036] 6-OH-BDE-85 –logREC 20 The experimentally determined value is 9.77. The prediction steps based on the QSAR model are as follows:
[0037] -logREC 20 =24.22×QE occ (-8.8eV,O)+2.61×QE vac (3.8eV,H)+0.79×logK OW -15.96
[0038] =24.22×(0.502)+2.61×(3.016)+0.79×(7.18)-15.96
[0039] =9.75
Embodiment 3
[0041] 6-OH-BDE-87: Its h is calculated by Williams graph method i The value is 0.252<h*(warning value)=0.667, standard residual (SE)=1.722<3, indicating that this compound is within the application domain of the QSAR model, but the prediction applied to this compound should be vigilant. Three descriptors were calculated by using the HF / 6-31G** algorithm of Gaussian 03 and EPI Suite respectively.
[0042] 6-OH-BDE-87 –logREC 20 The experimentally determined value is 9.29. The prediction steps based on the QSAR model are as follows:
[0043] -logREC 20 =24.22×QE occ (-8.8eV,O)+2.61×QE vac (3.8eV,H)+0.79×logK OW -15.96
[0044] =24.22×(0.506)+2.61×(3.091)+0.79×(7.18)-15.96
[0045] =10.05
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com