Preparation method of biosensor for detecting L-histidine and application thereof
A biosensor and histidine technology, applied in the field of analytical chemistry and biosensors, can solve the problems of needs, complex methods, and high detection costs, and achieve the effects of cheap price, simple operation, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
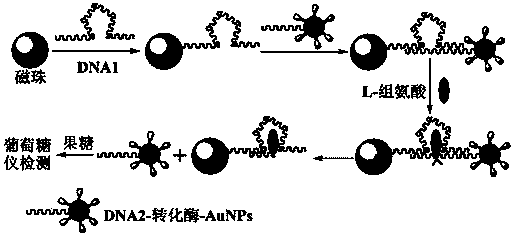
[0026] The present invention is achieved through the following measures: a preparation method of a biosensor for detecting L-histidine, comprising the following steps:
[0027] (1) Utilize existing method, prepare the colloidal gold of suitable particle size;
[0028] (2) Utilizing the effect of invertase and colloidal gold, the invertase is modified on the surface of colloidal gold;
[0029] (3) Utilize the effect of DNA2 and invertase-colloidal gold to modify the sulfhydryl-modified DNA2 onto invertase-colloidal gold;
[0030] (4) Using DNA1 and streptavidin-modified magnetic beads to modify DNA1-streptavidin on the magnetic beads to obtain DNA1-streptavidin-modified magnetic beads (DNA1-MBs);
[0031] (5) Using hybridization technology to construct biosensors.
[0032] The preparation of described DNA2-modified invertase-colloidal gold, DNA1-streptavidin-modified magnetic beads and sensors comprises the following steps:
[0033] (1) Activate 3.0 μmol of thiol-modified DNA2...
Embodiment
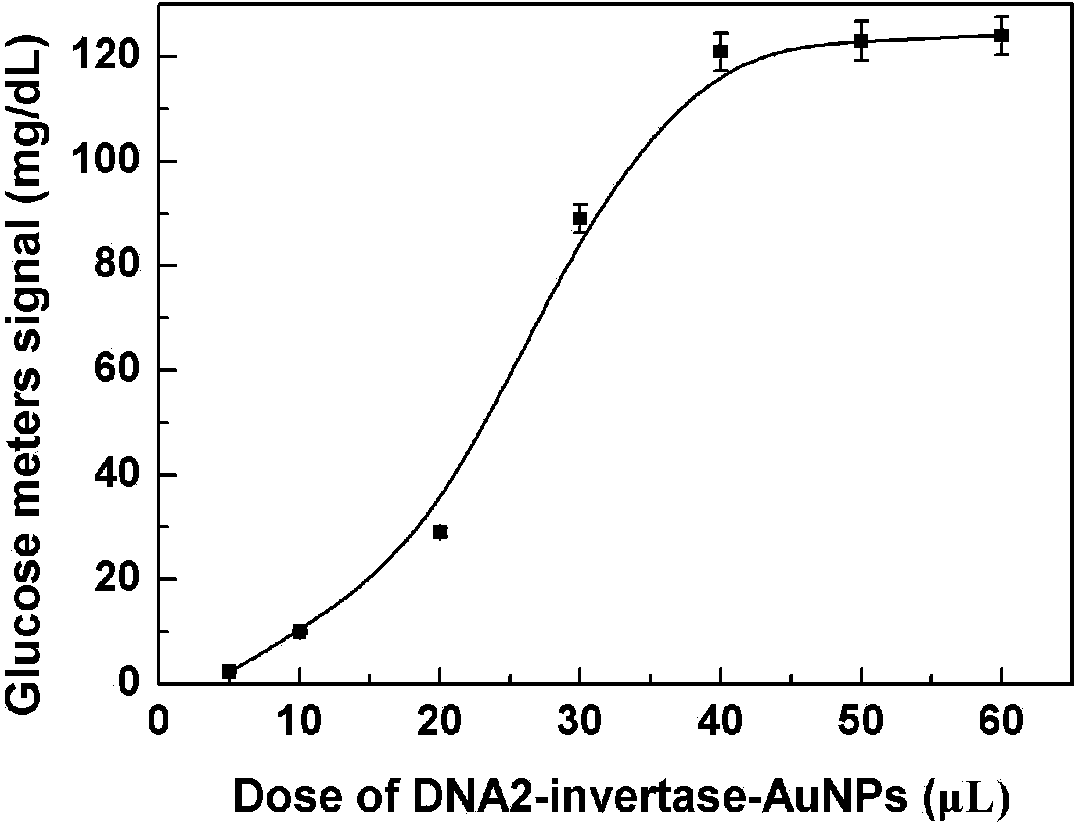
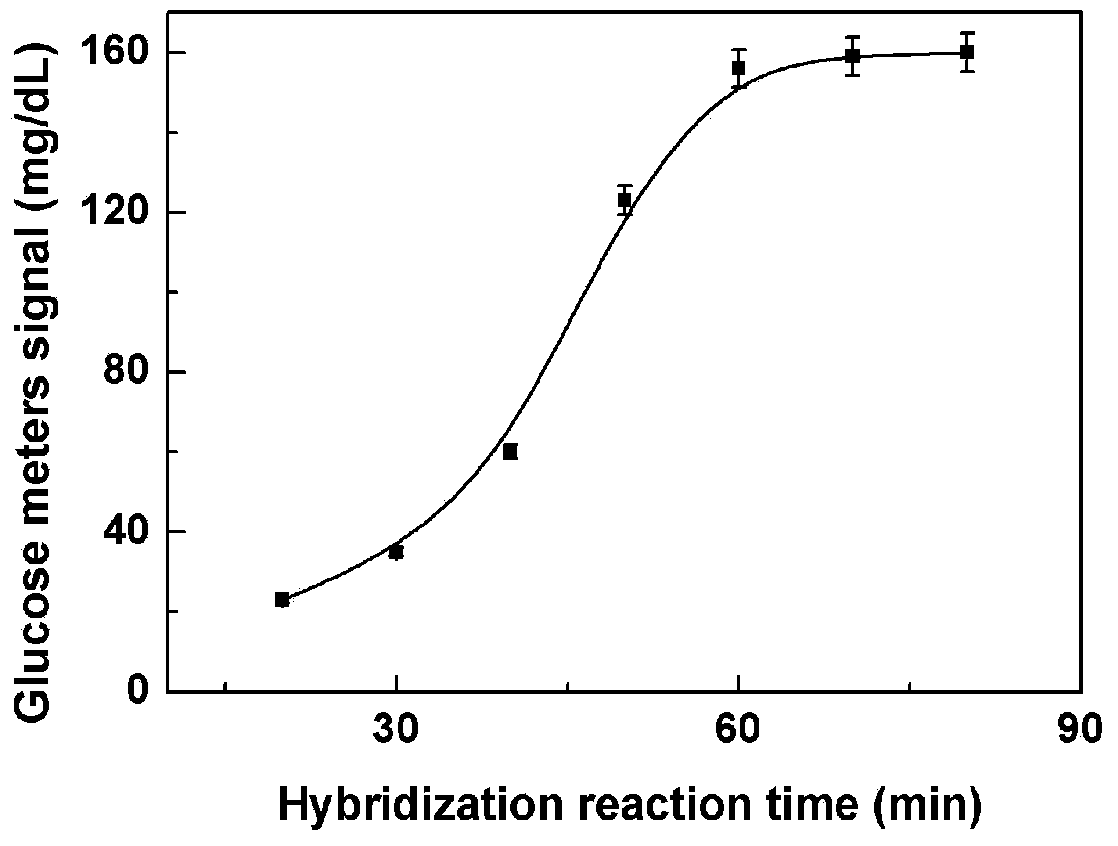
[0041] Embodiment: Biosensor detection L-histidine based on DNA hybridization technology
[0042] 1. Experimental part
[0043] 1.1 Instruments and reagents
[0044] 1.1.1 Instruments
[0045] Yicheng Blood Glucose Meter (Beijing Yicheng Bioelectronic Technology Co., Ltd.), Z-82A Air Bath Constant Temperature Oscillator (Quantan Medical Instrument Factory); Anke-TGL-16C Feiweng High-speed Centrifuge (Shanghai Anting Scientific Instruments) factory).
[0046] 1.1.2 Reagents
[0047] HAuCl 4 、Na 3 C 6 h 5 o 7 , TCEP (tris(2-carboxyethyl)phosphine hydrochloride), and sucrose were purchased from Sinopharm Chemical Reagent Co., Ltd.; invertase was purchased from Sigma; streptavidin-modified magnetic beads were purchased from Tianjin Beisi Le Chromatography Technology open your heart.
[0048] The DNA was synthesized by Saibaisheng Company, and the sequence is as follows:
[0049] DNA1:5'-GAG TGA TAT GAA GGA TGG GCG TGT CGG GGC TAT TCT CTA C-biotin-3'
[0050] DNA2:5'-GT...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com