A method for constructing an animal model for detecting allergens in food and its application
A technology of animal model and construction method, which is applied in the field of medical evaluation and detection, can solve the problems of not being a food allergen, easy to produce oral tolerance, etc., and achieve the effect of reducing risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Construction of animal models
[0104] 1. Experimental animals
[0105] SPF grade female Wistar rats weighing 40-60 g.
[0106] 2. Test substance
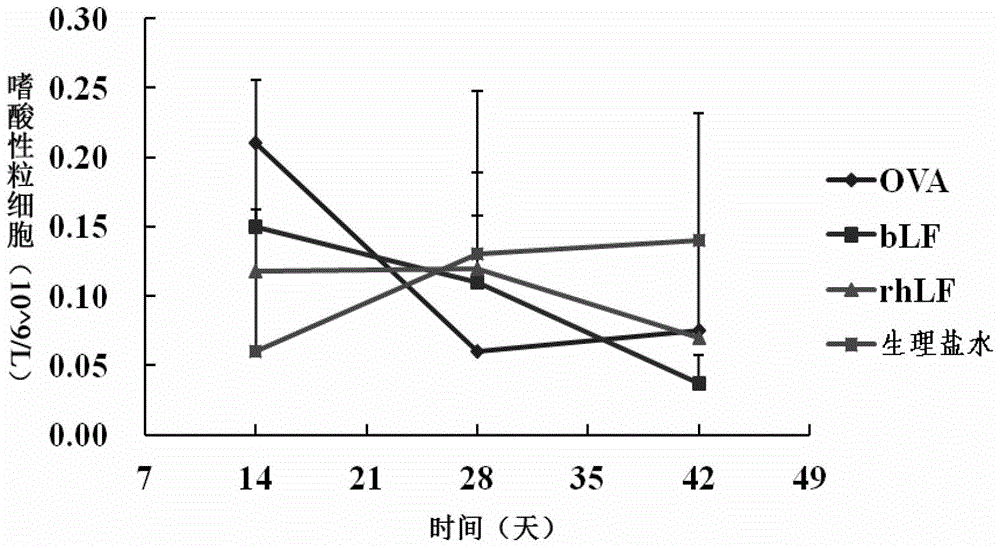
[0107] Human lactoferrin (rhLF), bovine lactoferrin (bLF), ovalbumin OVA, normal saline (negative control).
[0108] 3. Grouping of animals
[0109] Experimental animals were randomly divided into 4 groups according to body weight (two groups in each group), namely: rhLF gavage group / peritoneal injection group, bLF gavage group / peritoneal injection group, OVA gavage group / peritoneal injection group and normal saline gavage group group / peritoneal injection group. 8 animals per group.
[0110] 4. Dosage
[0111] rhLF and bLF: intragastric administration dose of 0.01g / kg body weight, intraperitoneal injection dose of 0.001g / kg body weight.
[0112] OVA: intragastric administration dose is 0.15g / kg body weight, intraperitoneal injection dose is 0.015g / kg body weight.
[0113] Normal saline: 8ml / kg body weight.
[0114] ...
Embodiment 2
[0121] Detection of reaction indicators
[0122] 1. Detection of specific IgE
[0123] 1) Coated
[0124] Dilute the test protein to 10 mg / L with coating buffer, select the wells to be coated on a 96-well plate, add 100 μL of coating solution to each well, and overnight at 4 °C;
[0125] 2) washing
[0126] Pour off the liquid in the wells, add 200 μL of washing solution to each well, place at room temperature for 3 minutes, shake off the washing solution, and pat it on absorbent paper several times until there is no obvious drop in the well, repeat twice;
[0127] 3) closed
[0128] Add 150 μL of blocking solution to each well, incubate for 1 h in a constant temperature incubator at 37 °C, and wash three times;
[0129] 4) Add primary antibody
[0130] Add 100 μL of serum to be tested diluted 1:50-100 to each well, make three parallel wells for each test serum, and make three negative controls on each plate. Incubate in a constant temperature incubator at 37°C for 1 hou...
Embodiment 3
[0163] Test Results and Analysis
[0164] 1. Daily situation
[0165] During the test period, the experimental animals in each group were in good condition, their nutritional status was normal, and no poisoning such as death occurred.
[0166] 2. Results of gavage group
[0167] 1) Specific IgE results
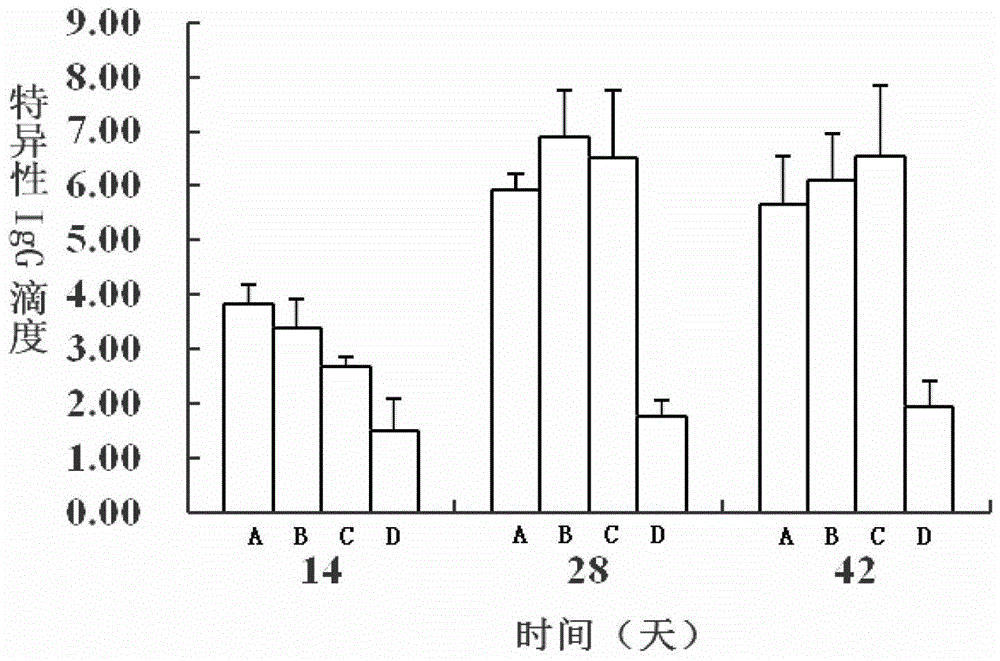
[0168] Table 1 shows the specific IgE detection results of each group in the intragastric administration group: it can be seen from the results that the OVA group was significantly higher than the bLF group on the 28th and 42nd days (P<0.05), and it was significantly higher on the 42nd day. higher than that of the normal saline control group (P<0.05). However, rhLF hardly induced IgE response in experimental animals.
[0169] Table 1 The specific IgE detection results of each group in the gavage group (mean ± standard deviation, n=8)
[0170]
[0171] Note: a indicates that there is a significant difference with the OVA group (P<0.05).
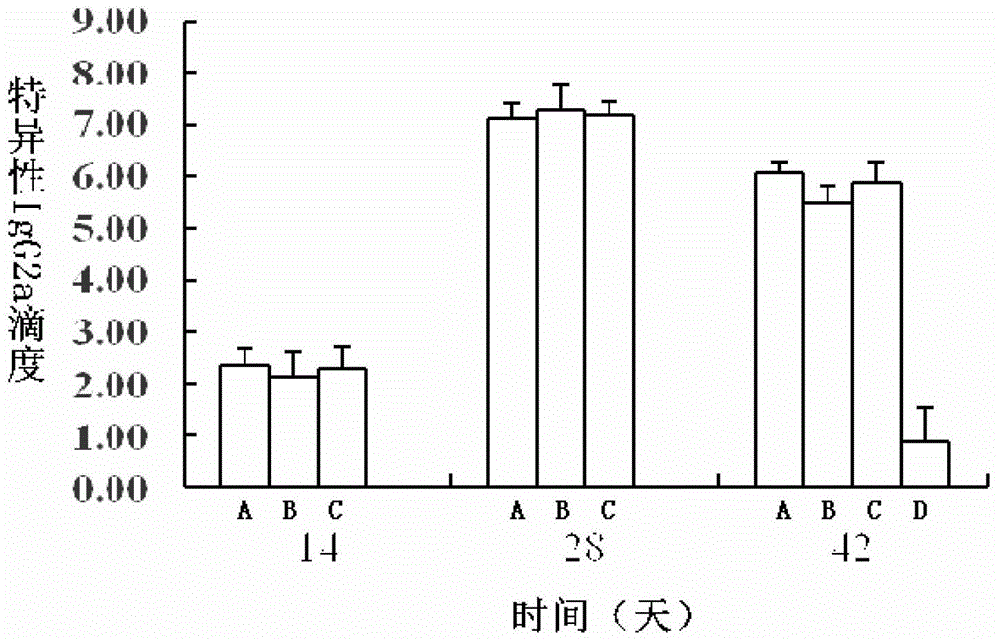
[0172] 2) Specific IgG results...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com