Crystallization methods for 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride
A technology for crude methoxypyridine hydrochloride and methoxypyridine hydrochloride, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of qualitative change in post-treatment, instability of omeprazole, etc., and achieve stable yield and method Simple and convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
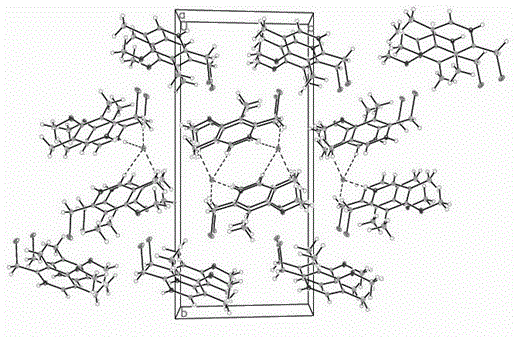
[0013] Take 1.00 g of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride, dissolve it completely with 15 mL of an equal volume mixture of ethanol and acetone, filter out impurities, and place it in a specific room After slow evaporation, 0.43 g of single crystal 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride was obtained after 48 h, with a yield of 43%. The crystalline crystals are monoclinic with space group P 2 1 / c , the unit cell parameters of the crystal: a = 6.074(11), b = 19.88(4), c = 9.230(16) ?, = 99.67(4) , V = 1098(3) ? 3 , Z = 4, M r = 221.10, D c = 1.343 g / cm 3 , (Mo K ) = 0.553 mm , F (000) = 464, S = 0.988. single molecule through hydrogen bonds to form a dimeric structure, such as figure 1 , figure 2 is the unit cell packing diagram formed by dimerized molecules.
[0014] Table 1
[0015]
[0016] Take 1.00 g of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride, dissolve it complet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com