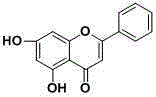
Application of chrysin in the preparation of anti-hypoxic drugs
A technology of chrysin and drug, applied in the field of medicine, can solve the problems of lack of anti-hypoxic injury protective drugs, no anti-hypoxia activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] tablet:
[0070] Chrysin (purity greater than 95%, Shaanxi Ciyuan Biotechnology Co., Ltd.) 50.0g,
[0071] Starch (filler, Shaanxi Aoke Pharmaceutical Excipients Co., Ltd.) 130.0g,
[0072] Microcrystalline cellulose (MCC, disintegrant, Shaanxi Aoke Pharmaceutical Excipients Co., Ltd.) 10.0g,
[0073] Methylcellulose (binder, Anhui Shanhe Pharmaceutical Excipients Co., Ltd.) 6.0g,
[0074] Magnesium stearate (lubricant, Shaanxi Aoke Pharmaceutical Excipients Co., Ltd.) 4.0g,
[0075] A total of 200.0g.
[0076] Prepared according to the conventional tablet method, and made 1000 tablets in total, each containing 50 mg of chrysin.
Embodiment 2
[0078] Capsules:
[0079] Chrysin (purity greater than 95%) 50.0g,
[0080] Starch (filler) 135.0g,
[0081] Methylcellulose (binder) 5.0g,
[0082] Magnesium stearate (lubricant) 10.0g,
[0083] A total of 200.0g.
[0084] Prepare according to the conventional method of capsules, and make 1000 capsules altogether, each capsule contains 50 mg of chrysin.
Embodiment 3
[0086] Dispersible tablets:
[0087] Chrysin (purity greater than 95%) 50.0g,
[0088] Microcrystalline Cellulose 130.0g,
[0089] Croscarmellose sodium (cCMC-Na, Anhui Shanhe Pharmaceutical Excipients Co., Ltd.) 20.0g,
[0090] A total of 200.0g.
[0091] Prepared according to the conventional method for dispersible tablets, a total of 1000 tablets were made, each containing 50 mg of chrysin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com