Medicine composition for treating urocystitis
A composition, the technology of cystitis, applied in the field of treating cystitis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
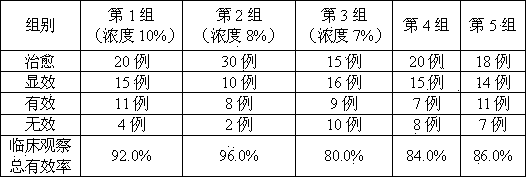
[0021] Example 1 Preparation of collagen perfusate
[0022] Prescription specifications: 50ml: 5g, concentration 10%
[0023] Collagen 100g Sodium chloride 8.5g Disodium phosphate 0.50g Sodium dihydrogen phosphate 0.04g Water for Injection Up to 1000ml (20 bottles)
[0024] Preparation: Make liquid under nitrogen-filled conditions, measure 90% volume of water for injection in the liquid mixing tank, weigh the prescription amount of sodium chloride, disodium hydrogen phosphate, and sodium dihydrogen phosphate, add it to the water, stir for 15 minutes to complete the dissolution Finally, add collagen, stir for 15 minutes to complete the dissolution, add water to the full volume, add 0.05% (w / v total volume) of activated carbon, absorb at 40-60°C for 15 minutes, decarbonize, and pass through a 0.22 μm filter membrane. Determination of intermediates, 95.0% of the labeled amount, so that the pH value is within the range of 7.2±0.1, filled ...
Embodiment 2
[0025] Example 2 Preparation of collagen perfusate
[0026] Prescription specifications: 50ml: 4g, concentration 8%
[0027] Collagen 80g Sodium chloride 8.5g Disodium phosphate 0.778g Sodium dihydrogen phosphate 0.06 Water for Injection Up to 1000ml (20 bottles)
[0028] Preparation: Make liquid under nitrogen-filled conditions, measure 90% volume of water for injection in the liquid mixing tank, weigh the prescription amount of sodium chloride, disodium hydrogen phosphate, and sodium dihydrogen phosphate, add it to the water, stir for 15 minutes to complete the dissolution Finally, add collagen, stir for 15 minutes to complete the dissolution, add water to the full volume, add 0.05% (w / v total volume) of activated carbon, absorb at 40-60°C for 15 minutes, decarbonize, and pass through a 0.22 μm filter membrane. Determination of intermediates is 105.0% of the labeled amount, so that the pH value is within the range of 7.2±0.1, filled...
Embodiment 3
[0029] Example 3 Preparation of collagen perfusate
[0030] Prescription specifications: 50ml: 3.5g, concentration 7%
[0031] Collagen 70g Sodium chloride 8.5g Disodium phosphate 0.50g Sodium dihydrogen phosphate 0.04g water for injection to 1000ml (20 bottles)
[0032] Preparation: Make liquid under nitrogen-filled conditions, measure 90% volume of water for injection in the liquid mixing tank, weigh the prescription amount of sodium chloride, disodium hydrogen phosphate, and sodium dihydrogen phosphate, add it to the water, stir for 15 minutes to complete the dissolution Finally, add collagen, stir for 15 minutes to complete the dissolution, add water to the full volume, add 0.05% (w / v total volume) of activated carbon, absorb at 40-60°C for 15 minutes, decarbonize, and pass through a 0.22 μm filter membrane. Determination of intermediates, 104.1% of the labeled amount, so that the pH value is within the range of 7.2±0.1, nitrogen ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com