Homogeneous luminescence immunoassay method for quantitatively analyzing multiple components simultaneously and kit used for method
A quantitative analysis and homogeneous luminescence technology, which is applied in the field of clinical examination and diagnosis, can solve the problems of complex, unsuitable and high detection cost of biochip technology, and achieve the effect of saving specimen consumption, simple operation and high precision.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
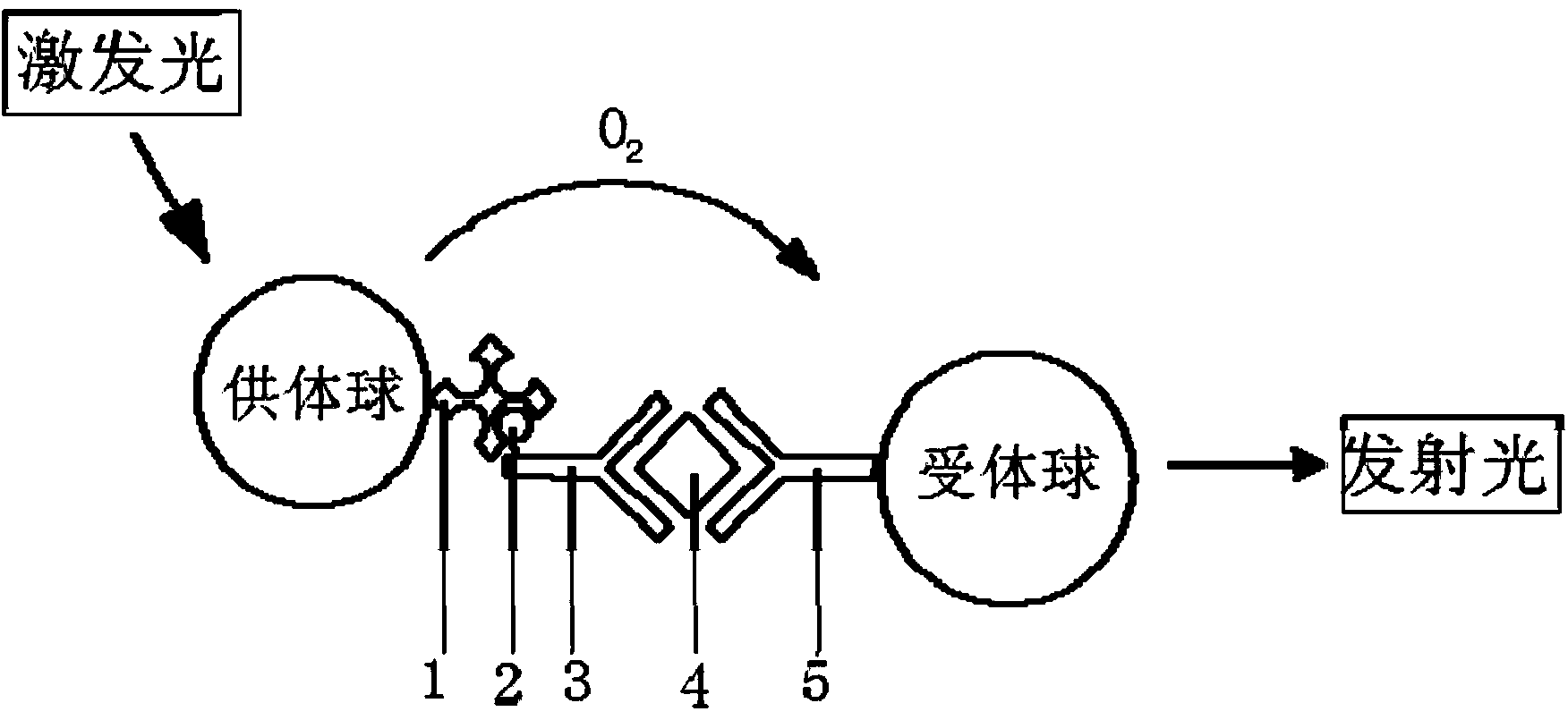
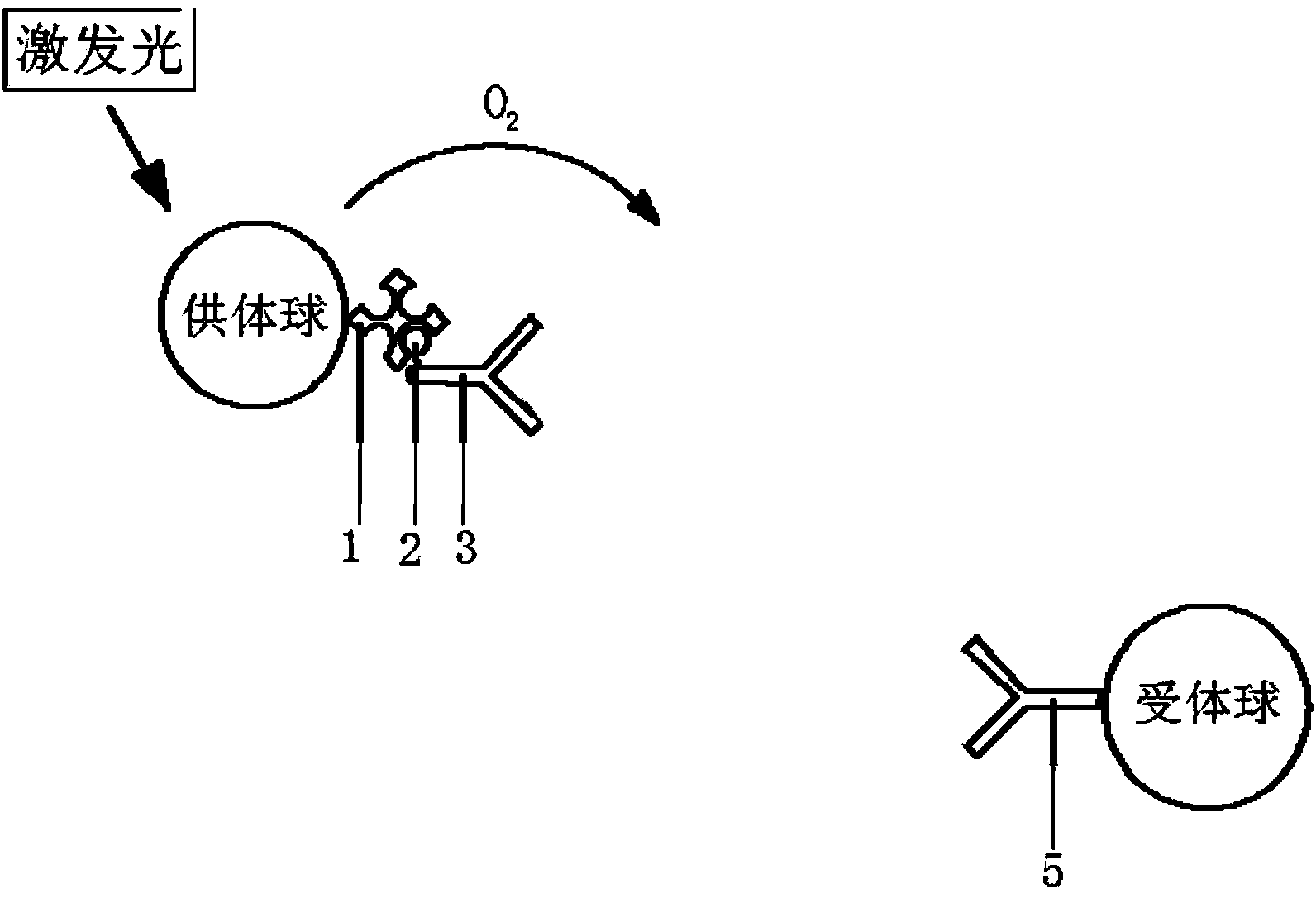
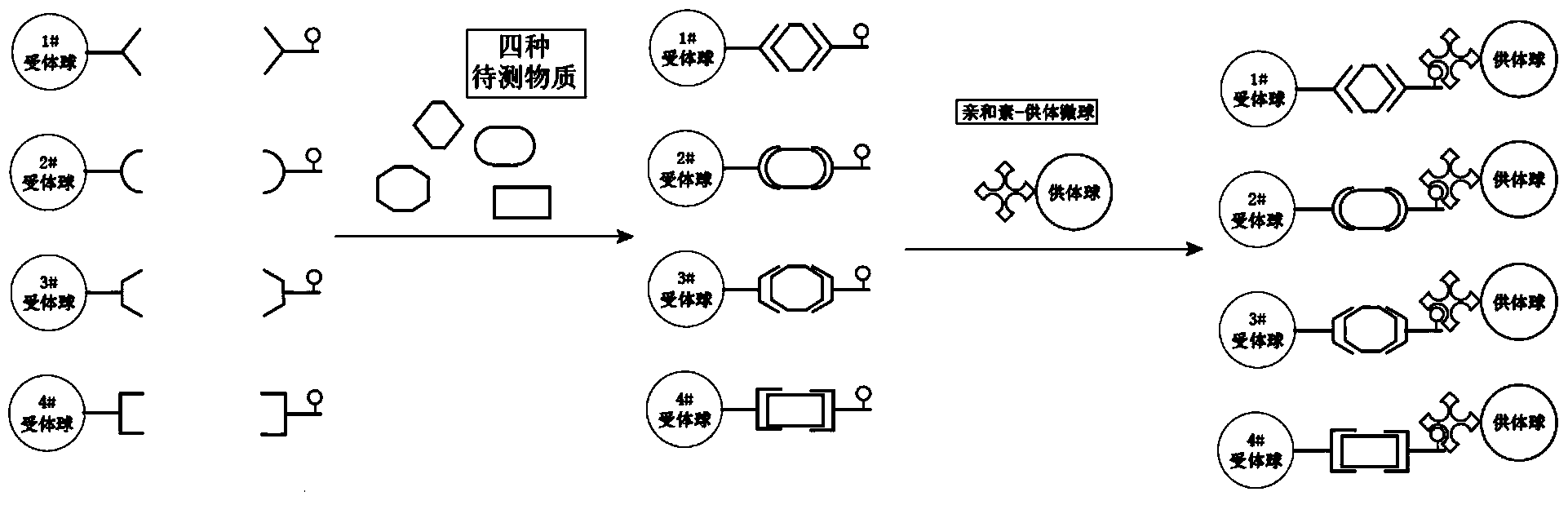
[0026] Taking the simultaneous quantitative analysis of serum alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), sugar chain antigen 125 (CA-125), and ferritin (Ferr) as examples, the homogeneous luminescence immunity for simultaneous determination of multiple components is explained in detail. Analysis methods (such as image 3 ): There are four types of acceptor microspheres in the detection system, all of which contain the same chemiluminescence agent and different fluorescein. Among them, 1# acceptor microspheres contain FITC and 2# acceptor microspheres contain PE, 3# receptor microspheres contain EDC, 4# receptor microspheres contain PE-Cy5), each receptor microsphere is coated with a polyclonal antibody, 1# receptor microsphere is anti-AFP, 2 #Receptor microspheres are anti-CEA, 3#receptor microspheres are anti-CA-125, and 4#receptor microspheres are anti-Ferr. In addition, there are four biotinylated monoclonal antibodies in the system, namely Bio-anti-AFP, Bio-a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com