Preparation method of streptococcus pneumoniae capsular polysaccharide protein conjugate vaccine
A protein-conjugated vaccine, Streptococcus pneumoniae technology, applied in the direction of antibacterial drugs, bacterial antigen components, etc., can solve the problem of measurement error and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] 7F Sonication:
[0083] a Weigh 250 mg of 7F polysaccharide, add dipure water to prepare a solution with a concentration of 2.5 mg / ml, and dissolve overnight at 4°C. In an ice bath, the solution was treated with an ultrasonic wave with a power of 80-100 W and a frequency of 20 kHz for 23 minutes.
[0084] b freeze-dried
[0085]c Using SEC-RI-MALLS combined technology with 0.2M NaCl as the mobile phase and SRT-SEC 1000A as the chromatographic separation column, the ultrasonically degraded polysaccharides were detected at a flow rate of 0.5 mL min-1 and a column temperature of 30 °C . The detectors are multi-angle laser detectors (MALLS) and differential refractometers (RI).
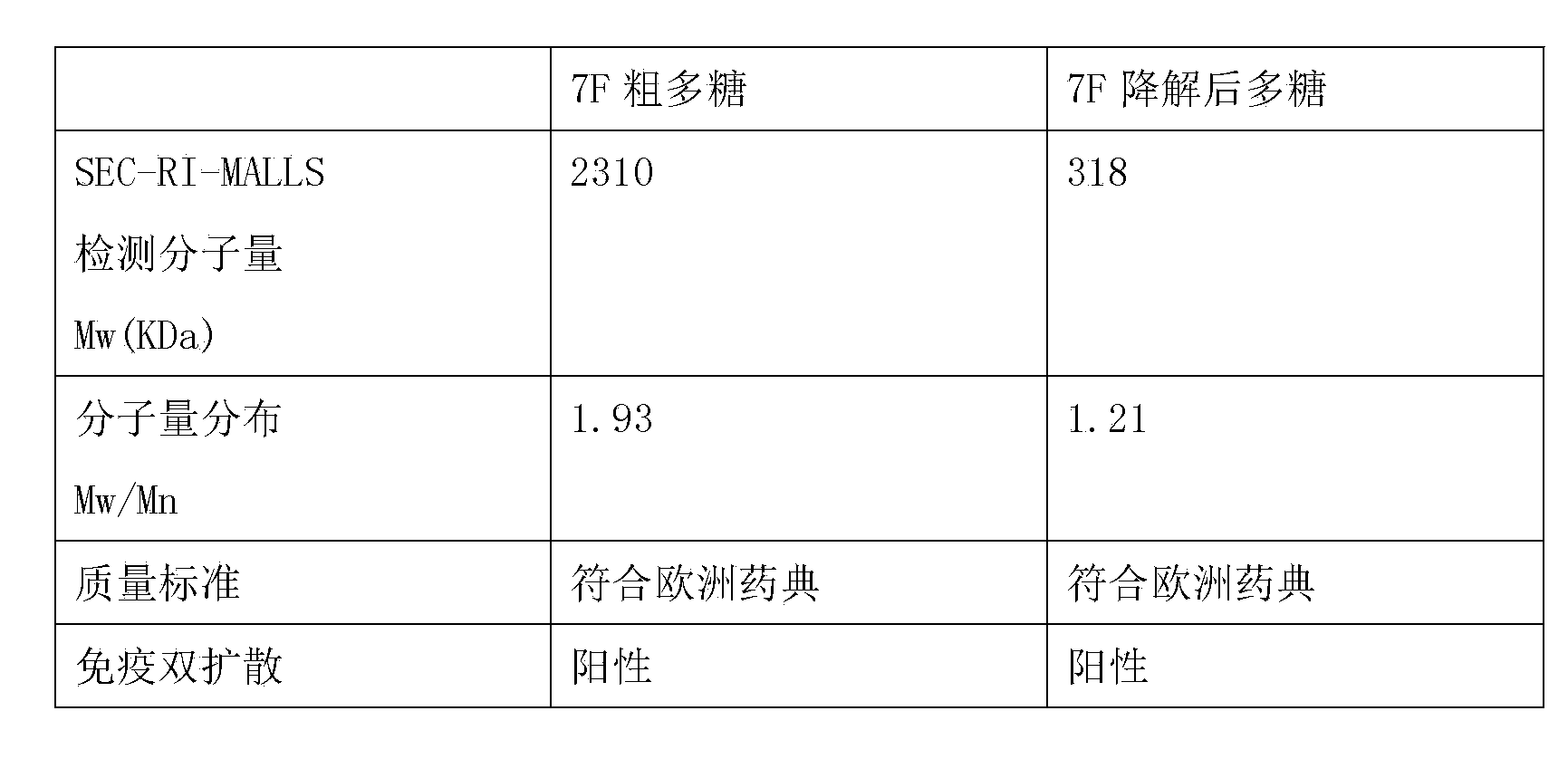
[0086] d The results are as follows
[0087] Table 1 Quality comparison of type 7F polysaccharides before and after degradation
[0088]
[0089] It can be seen from Table 1 that the molecular weight of the 7F crude polysaccharide is greater than 2000KDa. After ultrasonic degradation, the m...
Embodiment 2
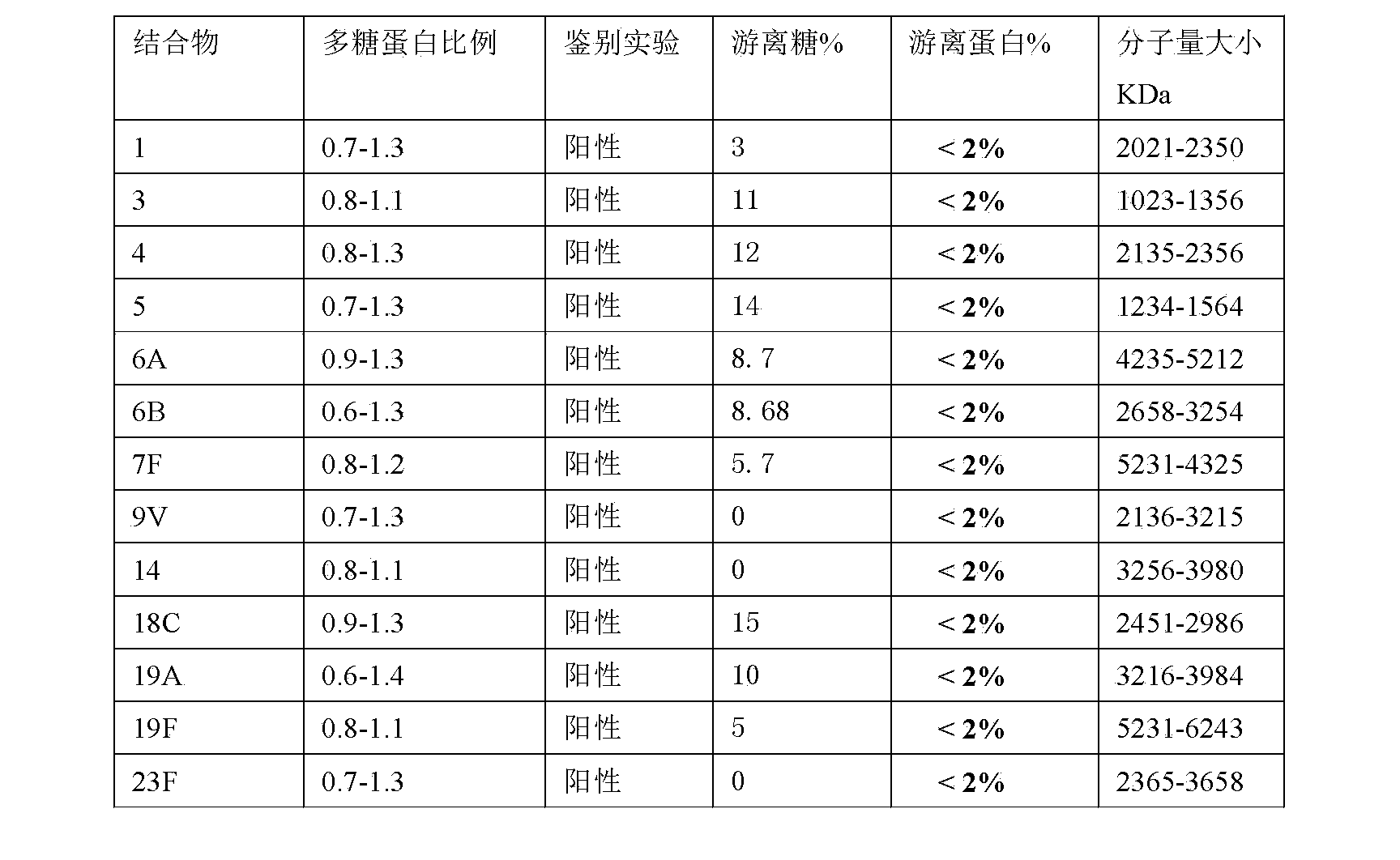
[0101] The method of Example 1 was used to prepare other 12 kinds of polysaccharide-protein conjugate vaccines except 7F.
[0102] The method is the same as in Example 1, but the polysaccharide is changed to one of 1, 3, 4, 5, 6A, 6B, 9V, 14, 18C, 19A, 19F or 23F
Embodiment 3
[0104] Quality control of the monovalent pneumonia polysaccharide protein conjugate obtained in Examples 1 and 2
[0105] The detection method of monovalent conjugate stock solution is as follows:
[0106] a molecular weight
[0107] Using SEC-RI-MALLS combined technology with 0.2M NaCl as the mobile phase and SRT-SEC 1000A as the chromatographic separation column, the molecular weight of the conjugate stock solution was detected at a flow rate of 0.5 mL min-1 and a column temperature of 30 °C . The loading volume is 100ul. The detectors are multi-angle laser scattering detectors (MALLS) and differential refractometers (RI).
[0108] b Determination of protein content
[0109] The BCA method was used to determine the protein content in the conjugate stock solution. A standard curve was drawn with BSA as the standard protein, and the protein content in the conjugate stock solution was calculated.
[0110] c Determination of polysaccharide content
[0111] The polysacchar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com