Application of unilaterally-substituted phenyl urea compound
A compound, phenylurea technology, applied in the preparation and application of organic compounds, and the preparation of urea derivatives, etc., can solve the problems of low contact activity, single modified skeleton, and inability to completely change defects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
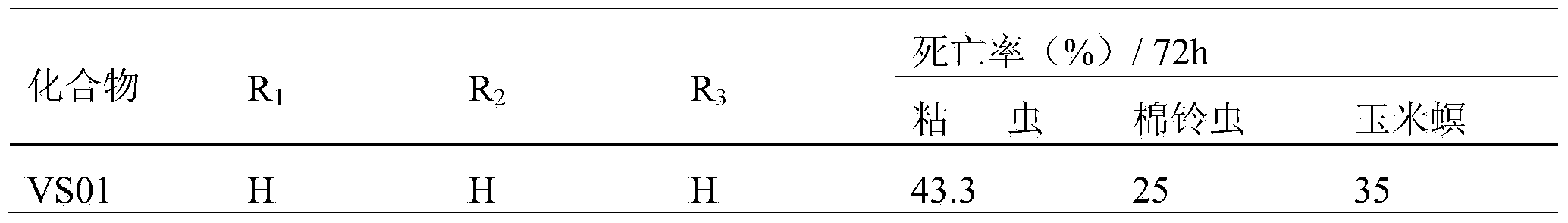
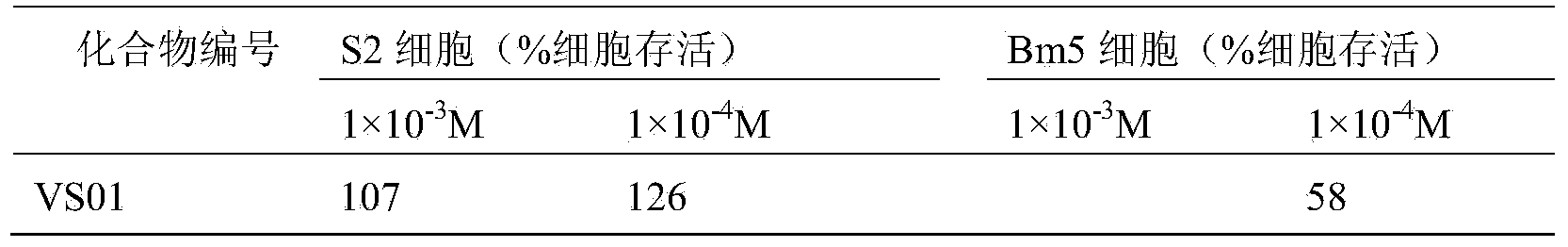
[0011] Experimental test insects: armyworm (Mythimna separata Walker), cotton bollworm (Helicoverpa armigera), and corn borer (Ostrinia nubilalis Hubner) were collected from normal groups raised indoors, and the third-instar larvae with the same age, weight and physiological status were used for chemical activity screening test.
[0012] Experimental method: Refer to the requirements of the "Standard Operating Specification (SOP) for Determination of Pesticide Biological Activity" provided by the subject, using the leaf soaking method, using corn leaves to soak in the liquid medicine prepared with acetone (600ppm, 600mg / L), and wait for the liquid to dry Inject 3rd instar larvae, mainly for stomach poisoning and contact killing, and observe the feeding phenomenon of larvae at the same time. 72-hour check for mortality.
[0013] The criteria for judging the death are: lightly touch the insect body, and the individual pests that cannot crawl normally are regarded as dead indivi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com