Pharmaceutical composition comprising fexofenadine
A technology of fexofenadine hydrochloride and its composition, which is applied in the field of stable pharmaceutical compositions of fexofenadine hydrochloride, can solve the problems of improving the storage stability and shelf life of pharmaceutical compositions without record
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
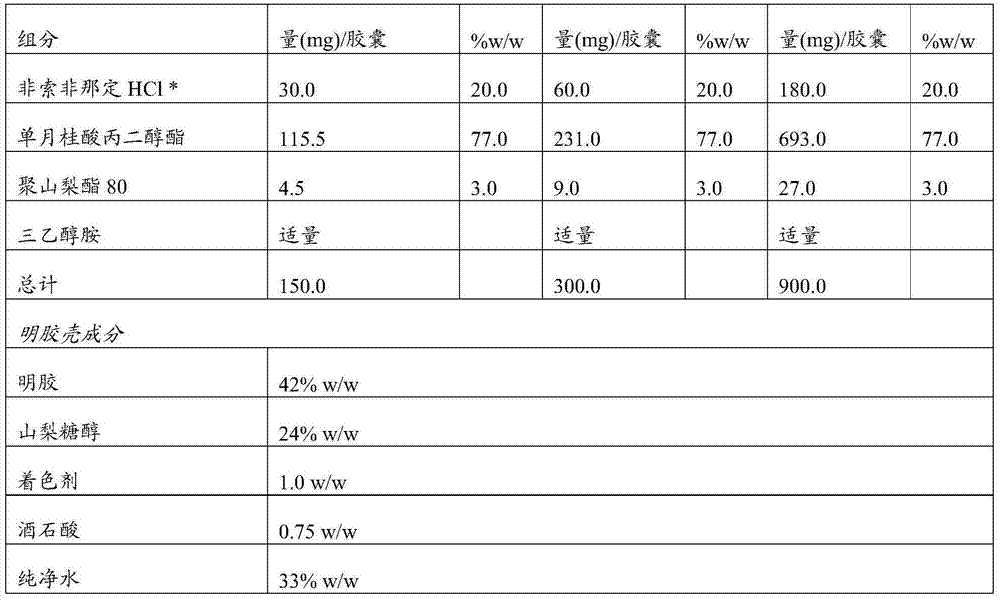
[0072] Example 1: Compositions of the invention
[0073]
[0074] *The specific surface area is 3.2m 2 / g
[0075] Disperse all excipients. Fexofenadine hydrochloride was dispersed with polysorbate 80 in propylene glycol monolaurate (glyceryl laurate) (under continuous stirring). The mixture was stirred for 45 minutes. The pH of the resulting mixture was adjusted to pH 5-6 with triethanolamine if necessary. The formulation is encapsulated in soft gelatin capsules with a fill weight of 900 mg (for 180 mg strength) according to one of the methods known to those skilled in the art.
Embodiment 2
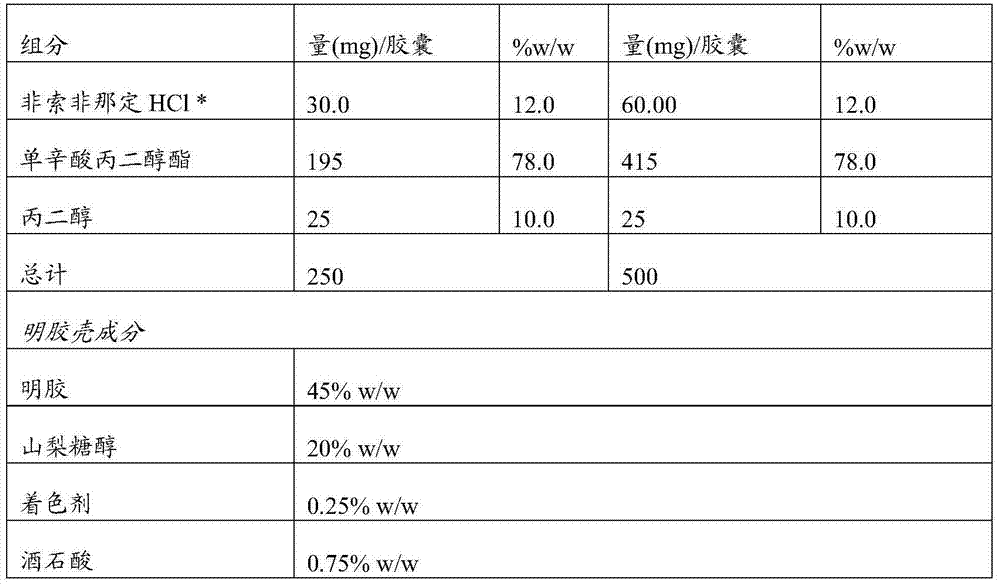
[0076] Example 2: Compositions of the invention
[0077]
[0078]
[0079] *The specific surface area is 3.2m 2 / g
[0080] Disperse all excipients. Fexofenadine hydrochloride was dispersed together with propylene glycol monocaprylate (propylene glycol caprylate-90) by heating to 125°C-165°C under continuous stirring until a clear solution formed. The resulting mixture was cooled to room temperature. The formulation is encapsulated in soft gelatin capsules according to one of the methods known to those skilled in the art.
Embodiment 3
[0081] Embodiment 3: Stability study of the composition of the present invention
[0082] The tested pharmaceutical composition for oral administration is based on the following formula (Fill Composition A):
[0083] Element
Features
Mg / capsule
%w / w
Fexofenadine HCL*
Active ingredients
180.00
30.0
Glyceryl Laurate-90
lipophilic surfactant
384.00
64.0
Tween 80
Hydrophilic Surfactant
36.00
6.0
Triethanolamine
pH regulator
Appropriate to pH5-6
total weight
600mg
[0084] *The specific surface area is 3.2m 2 / g
[0085] production method:
[0086] 1. Mix fexofenadine, glyceryl laurate 90, and Tween 80 in a stainless steel container for 15 minutes;
[0087] 2. Use a sufficient amount of triethanolamine to adjust the pH to 5-6;
[0088] 3. using one of the methods known to those skilled in the art to fill soft gel capsules with the mixture obtai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com