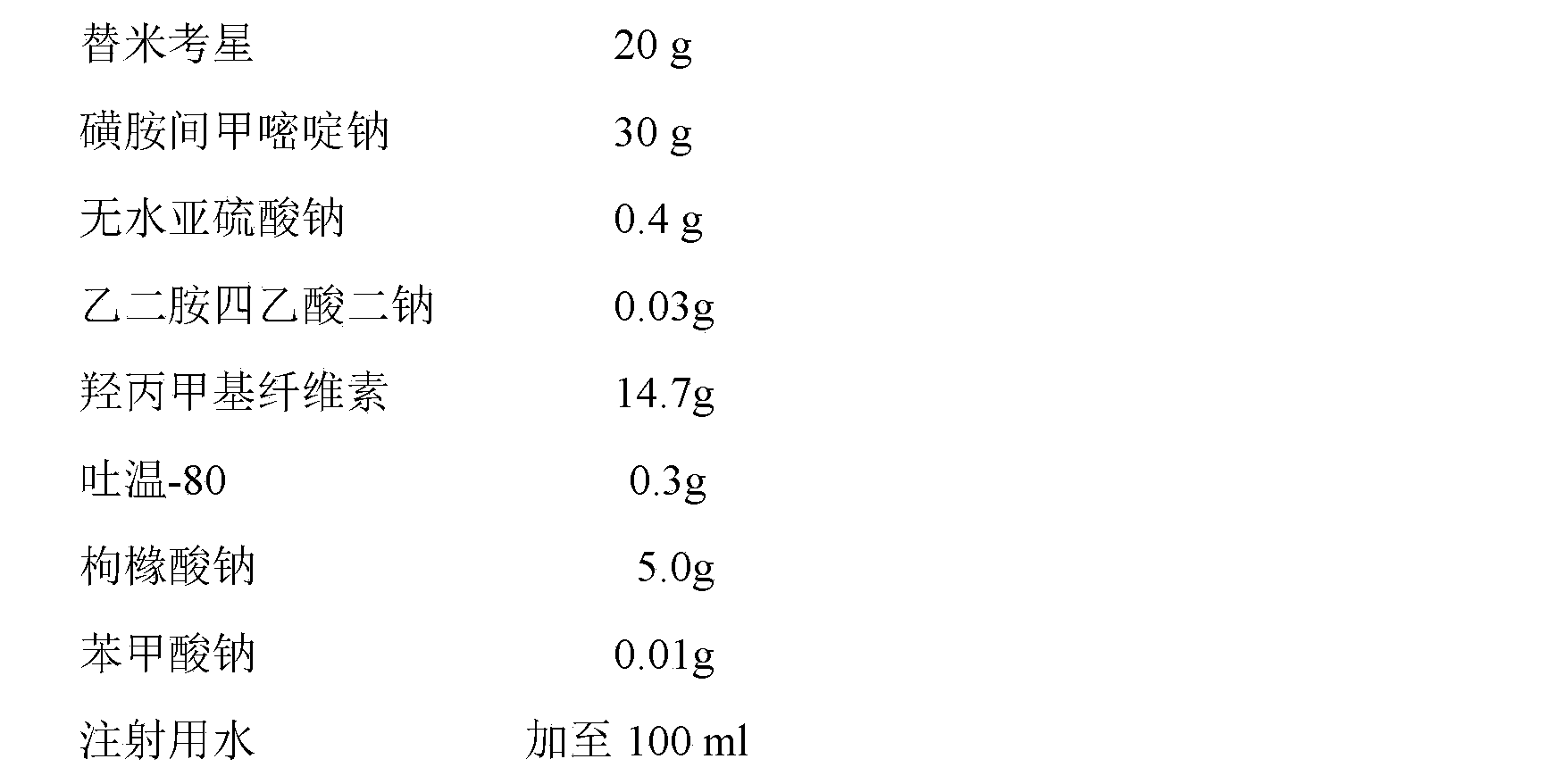Injectable composition containing tilmicosin and sulfonamides, and preparation process
A technology of sulfonamide drugs and tilmicosin, applied in the field of drugs, can solve the problems of inappropriate and unstable sulfonamide, and achieve the effects of reducing animal pain, low cost, reducing adverse reactions and other safety accidents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Component
[0031]
[0032] The preparation process is:
[0033] (1) First dissolve disodium edetate, sodium citrate and sodium benzoate in 20ml water for injection in sequence, and filter with 0.45μm microporous membrane to obtain solution A.
[0034] (2) Take 10ml of water for injection, dissolve in sodium carboxymethylcellulose and heat to dissolve, then add anhydrous sodium sulfite and Tween-80, stir to dissolve completely, filter with 0.45μm microporous membrane to obtain solution B.
[0035] (3) Pour solution B into solution A, stir and mix well, add sulfamethazine sodium and tilmicosin, and dilute to 100% (V / V) with water for injection to obtain mixed solution C.
[0036] (4) The mixture C is filtered and sterilized through a 0.22 μm microporous membrane, and filled aseptically to obtain Tilmicosin and Sulfamonomethazine Sodium Injection.
Embodiment 2
[0038] Component
[0039]
[0040]
[0041] The preparation process is:
[0042] (1) First dissolve disodium ethylenediaminetetraacetic acid, sodium citrate, potassium dihydrogen phosphate, and sodium formaldehyde sulfoxylate in 20ml water for injection, and filter with 0.45μm microporous membrane to obtain solution A.
[0043] (2) Take 10ml of water for injection, add anhydrous sodium sulfite to dissolve it, leave it at room temperature, add 50ml of water for injection, dissolve in sodium carboxymethyl cellulose and stir evenly, filter with 0.45μm microporous membrane to obtain solution B .
[0044] (3) Pour solution B into solution A, stir and mix well, add sulfamethazine sodium and tilmicosin, and dilute to 100% (V / V) with water for injection to obtain mixed solution C.
[0045] (4) The mixture C is filtered and sterilized through a 0.22 μm microporous membrane, and filled aseptically to obtain Tilmicosin and Sulfamethazine Sodium Injection.
Embodiment 3
[0047] Component
[0048]
[0049] The preparation process is:
[0050] (1) First dissolve disodium edetate, sodium citrate, potassium dihydrogen phosphate and sodium formaldehyde sulfoxylate in 20ml of water for injection, and filter with 0.45μm microporous membrane to obtain solution A.
[0051] (2) Take 10ml of water for injection, add sodium benzoate; then add 50ml of water for injection, dissolve sodium carboxymethyl cellulose and add Tween-80, after stirring, filter with 0.45μm microporous membrane to obtain solution B .
[0052] (3) Pour solution B into solution A, stir and mix well, add sulfamethazine and tilmicosin, and dilute to 100% (V / V) with water for injection to obtain mixed solution C.
[0053] (4) The mixture C is filtered and sterilized through a 0.22 μm microporous membrane, and filled aseptically to obtain Tilmicosin and Sulfamethazine injections.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com