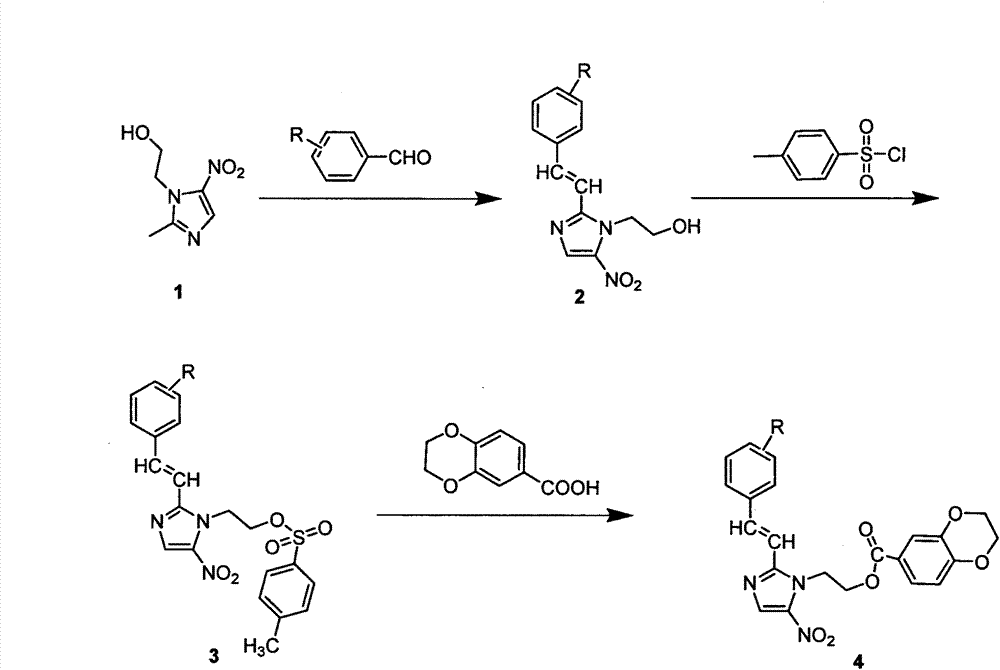
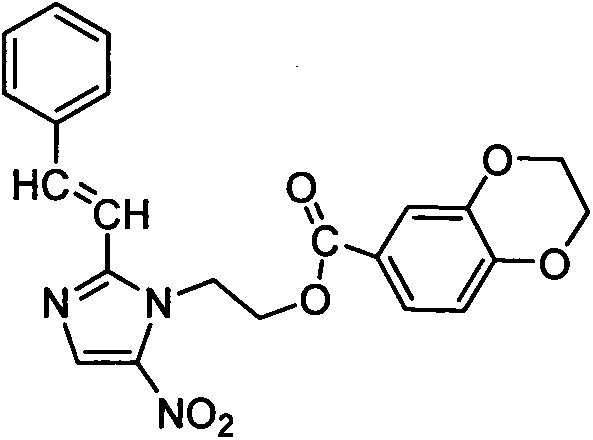
Synthesis and preparation of nitroimidazole derivative containing 1,4-benzdioxan skeleton and application of nitroimidazole derivative in anticancer drugs
A technology of benzodioxane and nitroimidazole is applied in the synthesis and preparation of a class of nitroimidazole derivatives containing 1,4-benzodioxane skeleton and its application in anticancer drugs, It can solve the problems of obvious adverse reactions and poor pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1: Preparation of (E)-2-(2-styryl-5-nitroimidazole)ethyl-4-methylbenzenesulfonate (3)
[0013]
[0014] Under stirring conditions, (E)-2-(2-styryl-5-nitroimidazole) ethanol (2.59 g, 10 mmol), 20 mL of CH 2 Cl 2 , p-toluenesulfonyl chloride (1.90g, 10mmol) and 10mL of triethylamine, stirred and reacted at room temperature for 7 hours (TLC detection reaction), filtered, washed and dried to obtain a crude product, and then the crude product was reconstituted in ethanol Crystallization gave the target compound as white crystals. Yield 83.5%.m.p.164~167℃; 1 H NMR (DMSO-d 6 , 300MHzδppm)δ: 8.01(s, 1H, C-CH-N), 7.91~7.74(m, 4H, Ph-H), 7.34~7.28(m, 5H, Ph-H), 7.04(d, J= 6.4Hz, 2H, C-CH-CH), 4.49(d, 2H, J=5.7Hz, C-CH 2 -C), 4.22(s, 2H, C-CH 2 -C), 2.44(s, 3H, CH 3 -C).
Embodiment 2
[0015] Example 2: (E)-2-(2-styryl-5-nitroimidazole) ethyl-2,3-dihydrobenzo[1,4]dioxane-6-carboxylate (4u ) preparation
[0016]
[0017] Add the above white product (2.06g, 5mmol) into a 25mL round bottom flask containing 15mL of DMF, stir until dissolved, then add 1,4-benzodioxane-6-carboxylic acid (1.7g, 7.5mmol), dissolve After adding K 2 CO 3 (5mmol, 0.69g) as catalyst. After reacting for 4 hours under reflux and stirring (TLC detection of reaction), let it stand until it cools down, then add the reactant to 20 mL of ice water, filter and dry the precipitated solid, and dissolve it with acetone and absolute ethanol for recrystallization. Yield 62.7%.m.p.173~175℃; 1 H NMR (DMSO-d 6 , 300MHz, δppm): 4.23(s, 2H, O-CH 2 -C), 4.36(s, 2H, C-CH 2 -O), 4.67(s, 2H, N-CH 2 -C), 5.01(s, 2H, C-CH 2 -O), 6.67(d, J=4.5Hz, 1H, C-CH-C), 7.23~7.35(m, 6H, C-CH-C, Ph-H), 7.45~7.55(m, 3H, Ph -H), 8.21(s, 1H, N-CH).
Embodiment 3
[0018] Example 3: (E)-2-(2-(2-fluorostyryl)-5-nitroimidazole)ethyl-2,3-dihydrobenzo[1,4]dioxane-6- Preparation of Carboxylate (4a)
[0019]
[0020] The preparation method is the same as that in Example 2, with (E)-2-(2-(2-fluorostyryl)-5-nitroimidazole)ethyl-4-methylbenzenesulfonate instead of (E)-2-( 2-Styryl-5-nitroimidazole)ethyl-4-methylbenzenesulfonate to give the yellow target compound. Yield 65.2%.m.p.176~177℃; 1 H NMR (DMSO-d 6 , 300MHz, δppm): 4.13(s, 4H, O-CH 2 -CH 2 -O), 4.58(t, J=6.2Hz, 2H, N-CH 2 -C), 5.02(s, 2H, C-CH 2 -O), 6.50(t, J=3.6Hz, 1H, C-CH-C), 6.91(d, J=8.4Hz, 1H, C-CH-C), 7.05~7.31(m, 5H, Ph- H), 7.79~7.95(m, 2H, Ph-H), 8.23(s, 1H, N-CH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com