Mycobacterium tuberculosis secretory protein PtpA capable of dephosphorylating p-JNK and p-P38
A technology of Mycobacterium tuberculosis, secreted protein, applied in the field of cell biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Mycobacterium tuberculosis secretory protein PtpA
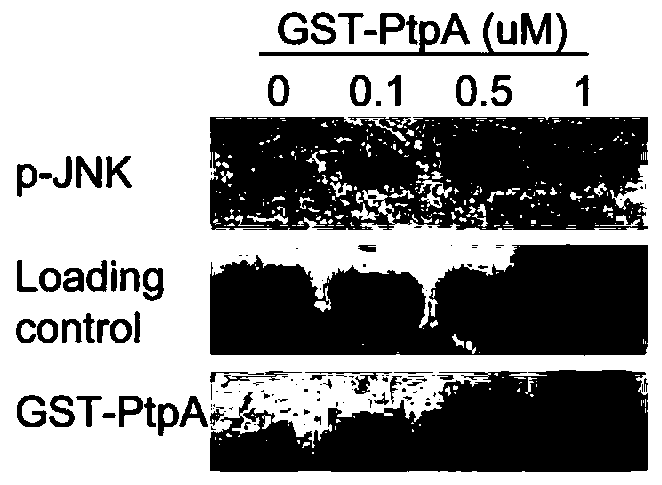
[0025] The amino acid sequence of the Mycobacterium tuberculosis secretory protein PtpA that can dephosphorylate host p-JNK and p-P38 proteins is shown in SEQ ID NO:1. PtpA and its structural analogues can be used in the development of JNK and P38 activity inhibitors.
Embodiment 2
[0026] Example 2 Ubiquitin molecules improve the phosphatase activity of PtpA
[0027] The protein molecule Ubiquitin (Ubiquitin), which can effectively improve the activity of the Mycobacterium tuberculosis secreted protein PtpA phosphatase, has an amino acid sequence as shown in SEQ ID NO:2. Ubiquitin and its structural analogues can be used in the development of PtpA phosphatase activity activators.
[0028] The ubiquitin protein preparation method used in this experiment is to construct the recombinant plasmid pET30a-Ubiquitin containing the Ubiquitin gene, and transform the plasmid into Escherichia coli BL21, induce protein expression with IPTG; ultrasonically break the bacteria, and collect the supernatant after high-speed centrifugation; Slowly flow the supernatant through the packed His-NTA nickel column, then add column washing buffer to fully wash the column, and finally add 2-3ml eluent to elute the protein; add the collected eluate to a 10KD Centrifuge and concent...
Embodiment 3
[0029] Example 3 PtpA and ubiquitin are applied to the dephosphorylation reaction of p-JNK and p-P38 protein
[0030] In vitro experimental system:
[0031] (A) Preparation of phosphatase PtpA protein
[0032] 1) Construct the recombinant plasmid pGEX-6P-1-PtpA containing the gene encoding PtpA, and transform the plasmid into Escherichia coli BL21;
[0033]2) Escherichia coli BL21 carrying the plasmid was added IPTG at 30°C to induce protein expression, and the induction time was 4 hours;
[0034] 3) Sonicate the bacteria, and collect the supernatant after high-speed centrifugation;
[0035] 4) Slowly flow the supernatant through the filled Glutathione-Sepharose beads (GE Company, GST column material), then add column washing buffer to fully wash the column, and finally add 2-3mL eluent to elute the protein;
[0036] 5) Add the collected eluate into a 30KD protein concentrator tube (millipore), and concentrate by centrifugation at 3500rpm for 20 minutes;
[0037] 6) Add 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com