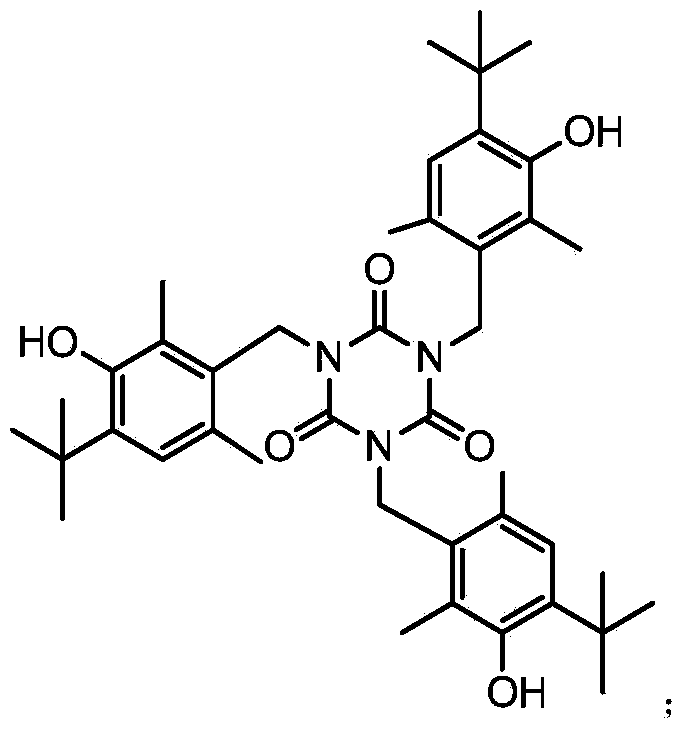
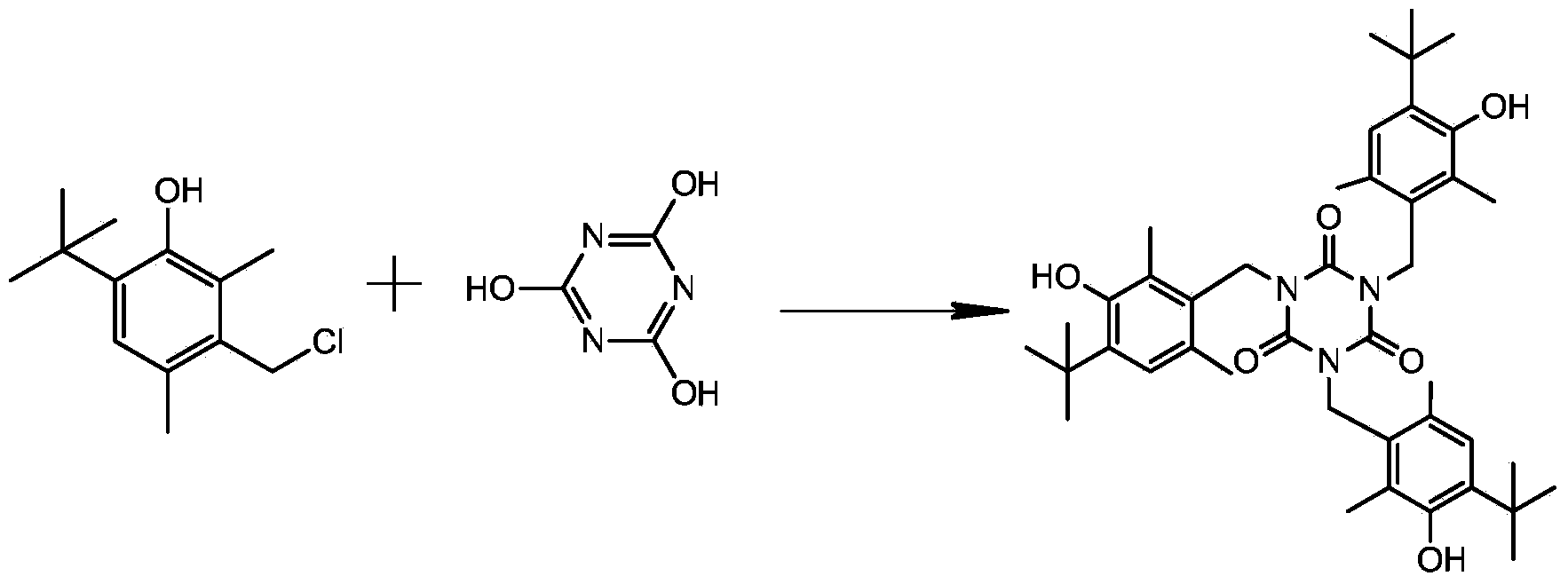
Synthesis method for antioxidant 1790
A synthetic method and anti-oxidant technology, applied in the direction of organic chemistry, etc., can solve the problems of unfriendly environment, long reaction time, increased cost, etc., and achieve the effect of favorable environment, low cost and less waste water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add 81g of N,N‐dimethylformamide and 16.2g (0.2mol) of potassium cyanate into a 500ml four-necked bottle, drop in 0.08g of tributylphosphine, protect it with nitrogen, heat to 80 degrees, add 22.3g (0.1mol) 2,6-dimethyl-4-tert-butyl-3-hydroxybenzyl chloride, after the addition, heat and react for 8 hours, remove the solvent under reduced pressure, slowly add water, stir to precipitate a solid, The crude product was obtained by filtration, and the filter cake was washed with water, and crystallized with ethanol and water to obtain 9.1 g of the product, yield: 35%
Embodiment 2
[0020] Add 162g of anhydrous N,N‐dimethylformamide and anhydrous 16.2g (0.2mol) potassium cyanate into a 500ml four-necked bottle, drop 0.3g of tributylphosphine, pass into nitrogen protection, and heat to At 130 degrees, add 45.3g (0.2mol) 2,6-dimethyl-4-tert-butyl-3-hydroxybenzyl chloride, heat the reaction for 8 hours after the addition, remove the solvent under reduced pressure, and slowly add water, Stir to precipitate a solid, filter, wash the filter cake with water, filter to obtain a crude product, and crystallize with ethanol and water to obtain 25 g of the product, with a yield of 47.8%.
Embodiment 3
[0022] Add 950g of N,N‐dimethylformamide to a 2L flask, then add 121g (1.5mol) of potassium cyanate, drop in 2g of tributylphosphine, heat to 95 degrees, then add 226.7g (1mol) of 2 , 6-dimethyl-4-tert-butyl-3-hydroxybenzyl chloride, heated for 9 hours after adding, removed the solvent under reduced pressure, slowly added water, stirred and precipitated solid, filtered to obtain crude product, used for filter cake After washing with water, recrystallize with ethanol and water, and dry to obtain 162.2 g of the product, with a yield of 62%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com