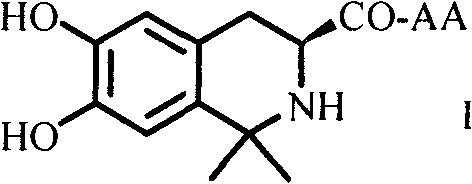
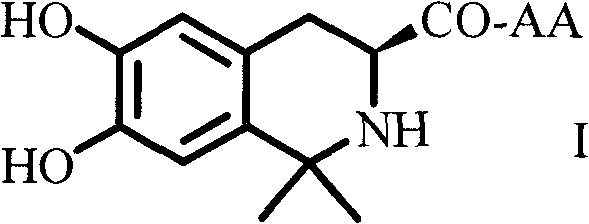
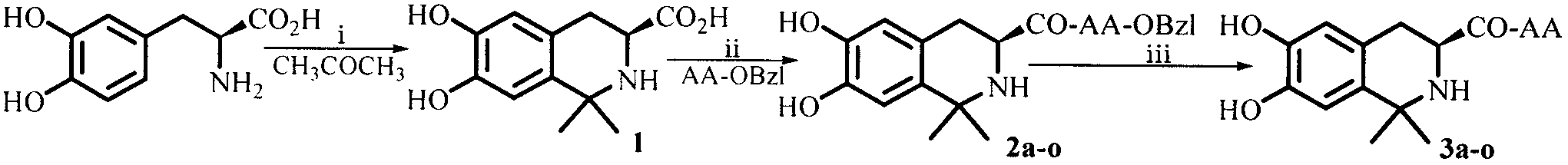Dihydroxyltetrahydroisoquinoline-3-formyl amino acids as well as synthesis, antithrombotic effect and application thereof
A kind of technology of tetrahydroisoquinoline and formyl amino acid, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 Preparation of (3S)-6,7-dihydroxy-1,1-dimethyl-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
[0016] 5.0 g (25 mmol) of 3,4-dihydroxy-L-phenylalanine were dissolved in 250 ml of acetone. To the resulting solution was added 6.0 g (30 mmol) of dried anhydrous magnesium sulfate. After stirring for 30 minutes, 25 ml of trifluoroacetic acid was added under ice cooling. The reaction mixture was then stirred at room temperature for 96 h, TLC (dichloromethane / methanol, 1:1) showed the disappearance of 3,4-dihydroxy-L-phenylalanine. The reaction mixture was filtered to remove solid magnesium sulfate. The filtrate was concentrated under reduced pressure on a water pump to remove acetone and trifluoroacetic acid. After the residue was a syrupy liquid, it was dissolved in acetone and continued to concentrate under reduced pressure, repeating 3 times. The residue was added with 200ml of anhydrous diethyl ether, a large amount of colorless solid was precipitated, and...
Embodiment 2
[0017] Example 2 Preparation of (3S)-6,7-dihydroxy-1,1-dimethyl-1,2,3,4-tetrahydroisoquinoline-3-formyl-L-Ala-OBzl (2a)
[0018] 616 mg (2.6 mmol) of (3S)-6,7-dihydroxy-1,1-dimethyl-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid were dissolved in 3 ml of anhydrous DMF. To the resulting solution was added 324 mg (2.4 mmol) of N-hydroxybenzotriazole. After 10 minutes, a solution of 500 mg (2.2 mmol) dicyclohexylcarbodiimide and 5 ml anhydrous DMF was added under ice cooling to obtain a reaction solution (I). 702 mg (2 mmol) of Tos·Ala-OBzl was dissolved in 5 ml of anhydrous DMF and stirred for 30 minutes to obtain a reaction solution (II). The reaction solution (II) was added to the reaction solution (I) under ice-cooling, and then stirred at room temperature for 12 h. TLC (dichloromethane / methanol, 6:1) showed that Tos·Ala-OBzl disappeared. The reaction mixture was filtered to remove dicyclohexylurea. DMF was purged from the filtrate at room temperature. The residue was ...
Embodiment 3
[0019] Example 3 Preparation of (3S)-6,7-dihydroxy-1,1-dimethyl-1,2,3,4-tetrahydroisoquinoline-3-formyl-Gly-OBzl (2b)
[0020] According to the method of Example 2, 616mg (2.6mmol) 3S)-6,7-dihydroxyl-1,1-dimethyl-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid and 674mg (2 mmol) Tos.Gly-OBzl yielded 250 mg (25%) of the title compound as a colorless powder. ESI-MS(m / e)85[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com