Acetophenone-substituted straight-chain sesqui derivative and application thereof in inhibiting basterium drug resistance
A technology of acetophenone and derivatives, applied in the field of pharmacy, can solve the urgent need for new antibacterial agents and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
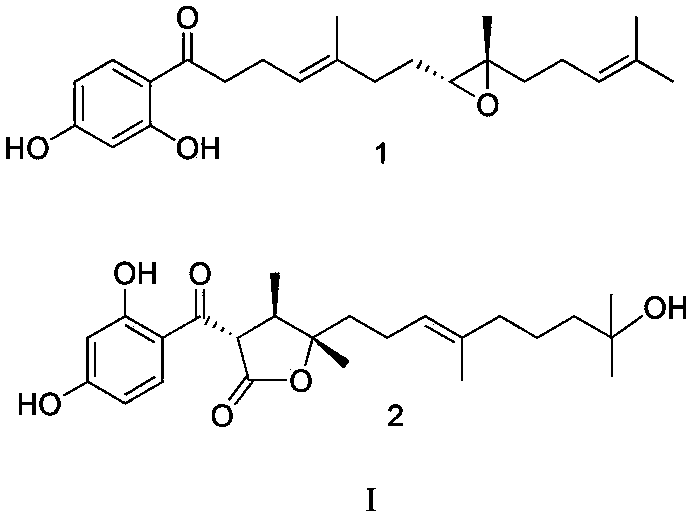
[0039] Example 1 Preparation of phenylpolyene sesquiterpene derivatives compounds 1 and 2 by ferulocarp
[0040] 2.0 kg of root parts of dried plant Ferula ferulaeoides (Steud.) Korov. were crushed and mixed with 20 times the amount of 95% ethanol at 45 o C soaking and extracting for 3 times, the extracts were combined and evaporated to dryness under reduced pressure to obtain extract, which was then extracted with dichloromethane 3 times, and evaporated to dryness under reduced pressure to obtain 400 g of extract. The sample was subjected to silica gel column chromatography, using 200-300 mesh silica gel (Qingdao Ocean Chemical Factory, China), petroleum ether-ethyl acetate (100:0 ~ 0:100) gradient elution, and silica gel thin-layer chromatography detection. Combine petroleum ether-ethyl acetate 70:30 eluted fractions, and repeatedly use 300-400 mesh silica gel column chromatography, the eluent is petroleum ether-acetone (85:15), to obtain compound 1 (22.0 mg); combine petro...
Embodiment 2
[0041] Embodiment 2 antibacterial experiment
[0042] Before the minimum inhibitory concentration (MIC) test, all test strains were inoculated on agar medium (Oxoid) at 37 o C cultured for 24 h. Control drugs Tetracycline, nor?oxacin, erythromycin and oxacillin were purchased from Sigma Chemical Co. The matrix was cation-adjusted broth (Mueller-Hinton broth, MHB; Oxoid), adjusted for Ca 2+ and Mg 2+ The concentration is 20 mg / L. Prepare the inoculum concentration of each Staphylococcus aureus strain (S. aureus) as 5×10 5 cfu / mL. Tetracycline and oxacillin were directly dissolved in the broth. Norfloxacin, erythromycin and the compound dshamirone were first dissolved in DMSO and then diluted into the broth to prepare 512 μg / mL as the initial concentration. The antibacterial activity of the control drug and compound dshamirone was determined by 96-well plate method. Add 125 μL of bacterial inoculum to all wells of the microplate, and o C for 18 h. The results were obse...
Embodiment 3
[0044] Example 3 Compound external pump and toxicity test
[0045] In the external pump inhibition experiment, the bacterial strain was cultured in broth for 24 hours, then centrifuged and precipitated, and the supernatant was removed, added to the buffer solution containing glucose, and diluted to the required concentration with an absorbance meter; Spectrophotometer, time scanning method was used to measure the fluorescence intensity, drug was added after 10 min in the administration group, and the scan was continued for 10 min; no drug was added in the control group, and the inhibitory effect of compounds 1 and 2 on the ethidium bromide efflux pump was observed.
[0046] Cell count Kit-8 (Dojindo, Japan) was used in the cytotoxicity test. Trypsinized HEK293 cells were cultured on a 96-well plate. After 24 hours, the cells were inoculated into the medium containing the drug at a specific concentration and cultured for 24 hours. CCK-8 reagent was added, and the OD450 was meas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com