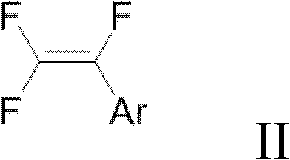
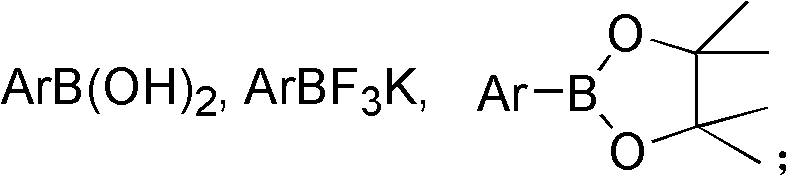Preparation method of trifluorostyrene compound
A technology of trifluorostyrene and compounds, which is applied in the field of new synthesis of trifluorostyrene compounds, can solve the problems of complex operation, low reaction selectivity, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Synthesis (I) of 1,2,2-trifluorostyrene: phenylboronic acid (122mg), potassium phosphate (636mg), bis(dibenzylideneacetone) palladium (1.2mg), tri-tert-butylphosphine tetrafluoro Add borate (1.2mg) into a 25ml Schlenk tube, repeat vacuuming, and fill with argon three times; and add 2ml redistilled toluene, 2ml redistilled DMF, 1.4ml deionized water under argon atmosphere, slowly Chlorotrifluoroethylene gas was introduced for 5min (excess). Then tighten the Teflon cock and heat at 80°C for 2h. After cooling, 1 mmol of fluorobenzene was added as an internal standard, and the yield of the fluorine spectrum was >99%. Much higher than the fluorine spectrum yield of about 70% in WO03051801.
[0121] Synthesis (II) of 1,2,2-trifluorostyrene: phenylboronic acid (122mg), potassium phosphate (636mg), bis(dibenzylideneacetone)palladium (1.2mg), tri-tert-butylphosphinetetrafluoro Add borate (1.2mg) into a 25ml Schlenk tube, repeat vacuuming, and fill with argon three times; and ...
Embodiment 2
[0123] Synthesis of 4-tert-butyltrifluorostyrene: 4-tert-butylphenylboronic acid (180 mg), potassium phosphate (636 mg), bis(dibenzylideneacetone) palladium (1.2 mg), tri-tert-butylphosphine tetra Add fluoroborate (1.2mg) into a 25ml Schlenk tube, repeat vacuuming, and fill with argon three times; and add 2ml redistilled DMF, 2ml redistilled toluene, 1.4ml deionized water under argon atmosphere, under stirring Chlorotrifluoroethylene gas was introduced slowly for 5min (excess). Then tighten the Teflon cock and heat at 80°C for 2h. After cooling, the reaction solution was washed three times with deionized water, then the aqueous phase was extracted three times with n-pentane, the organic phases were combined, and dried over anhydrous sodium sulfate. 100-200 mesh silica gel was added to the organic phase, spin-dried, and n-pentane column chromatography gave a colorless liquid with a yield of 94%. 1 H NMR (300.0MHz, CDCl 3 , 298K, TMS) δ7.42 (dd, J = 7.45, 7.40Hz, 4H), 1.33 (s...
Embodiment 3
[0125] Synthesis of 6-methoxy-2-(1,2,2-trifluorovinyl)naphthalene: 6-methoxy 2-naphthaleneboronic acid (202mg), potassium phosphate (636mg), bis(dibenzylidene Acetone) palladium (1.2mg), tri-tert-butylphosphine tetrafluoroborate (1.2mg) were added to a 25ml Schlenk tube, vacuum was repeated, and argon was filled three times; Re-distilled toluene, 1.4ml deionized water, and slowly introduce chlorotrifluoroethylene gas for 5min under stirring. Then tighten the Teflon cock and heat at 80°C for 2h. After cooling, the reaction solution was washed three times with deionized water, then the aqueous phase was extracted three times with n-pentane, the organic phases were combined, and dried over anhydrous sodium sulfate. The organic phase was added with 100-200 mesh silica gel, spin-dried, and subjected to petroleum ether column chromatography, the yield was 82%. 1 H NMR (399.6MHz, CDCl 3 , 298K, TMS) δ7.88(s, 1H), 7.77(d, J=8.7Hz, 1H), 7.76(d, J=8.9Hz, 1H), 7.52(d, J=8.7Hz, 1H), 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com