Preparation method of triclabendazole
A technology of triclabendazole and preparation steps, which is applied in the field of preparation of triclabendazole, and can solve problems such as environmental pollution, unsuitability for industrial production, difficulty in filtering iron sludge, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: The preparation steps of triclabendazole according to the present invention are as follows:
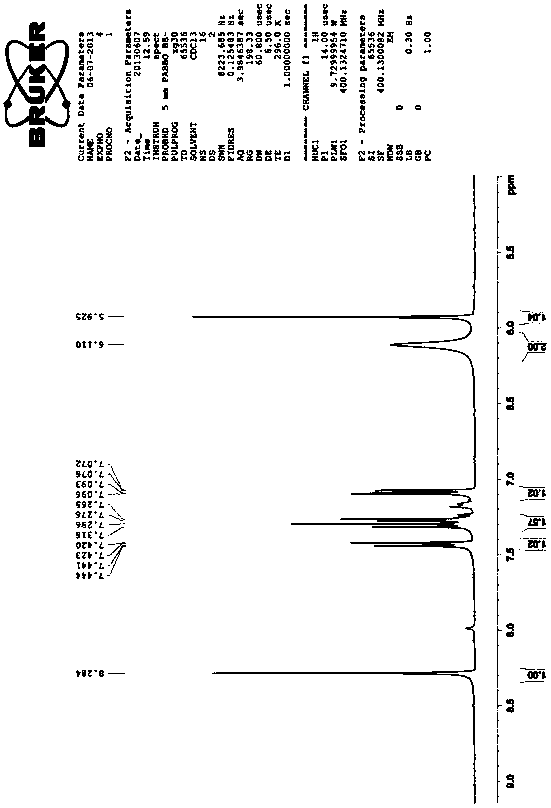
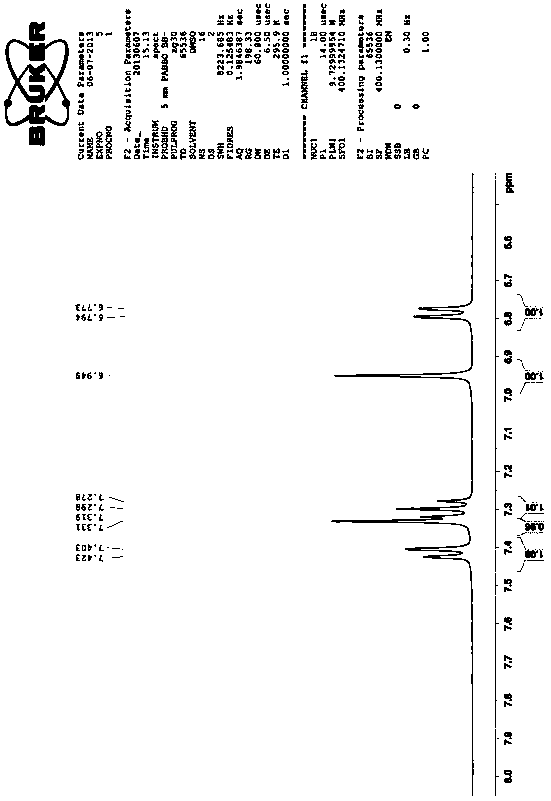
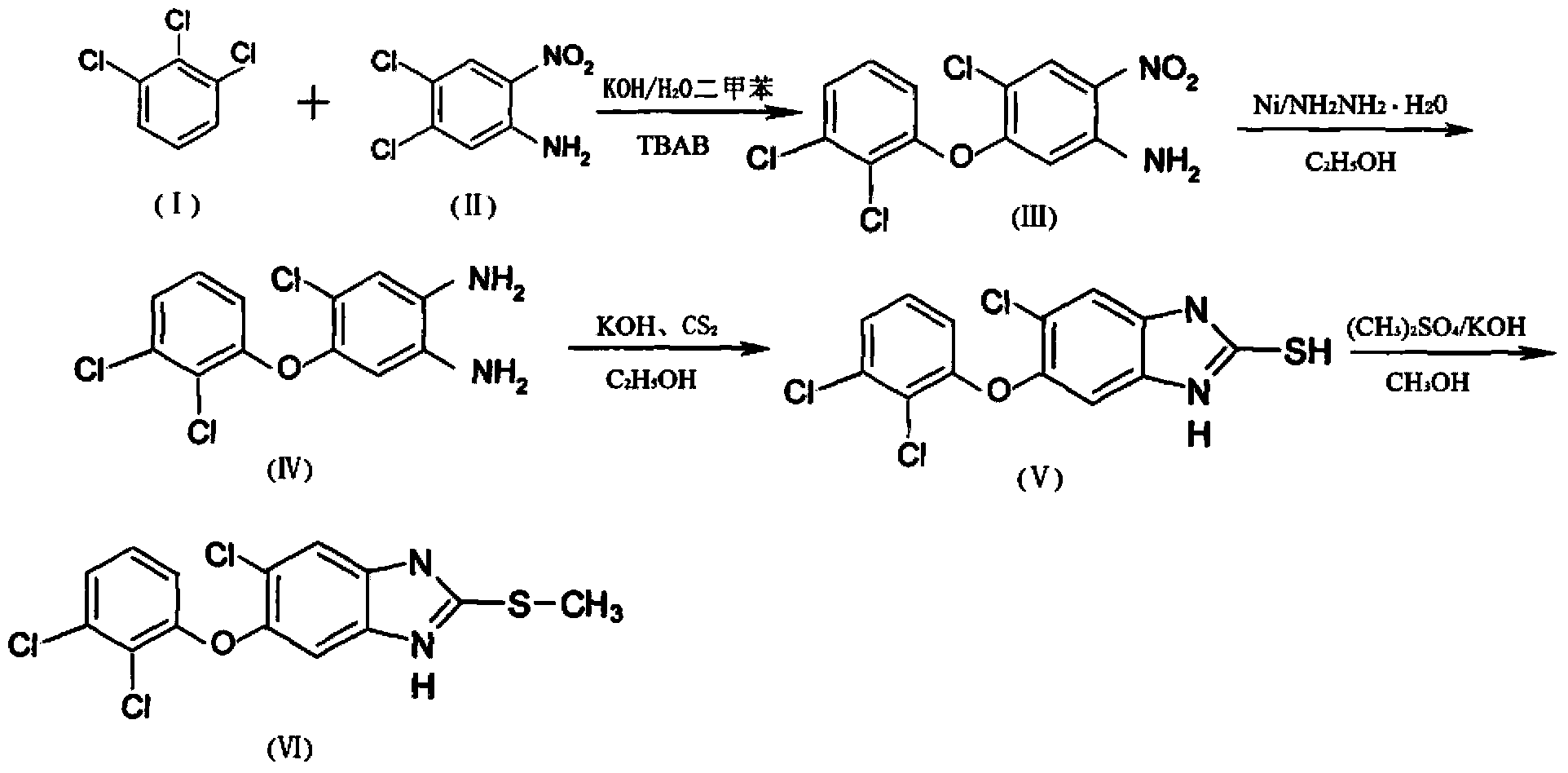
[0020] The first step, the preparation of 4-chloro-5-(2,3-dichlorophenoxy)-2-nitroaniline, that is, the compound III in the reaction formula:
[0021] Add 43.5kg of 1,2,3-trichlorobenzene and 40kg of 50% potassium hydroxide aqueous solution into the reaction kettle, heat and reflux for 7 hours, then add 150L of xylene and 41.4kg of 4,5-dichloro-2-nitroaniline React with 5 kg of catalyst TBAB for 8 hours, the reaction temperature is controlled at 125 ° C, slowly cooled to room temperature under stirring, a large number of brownish yellow crystals are precipitated, filtered, washed with 10 kg of frozen xylene, drained, washed with water until neutral, and dried to obtain 4-Chloro-5-(2,3-dichlorophenoxy)-2-nitroaniline 54kg, yield: 81%, melting point: 145°C-150°C, the spectrum of this substance is as follows figure 1 Shown; Wherein, 1,2,3-trichlorobenzene is the com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com