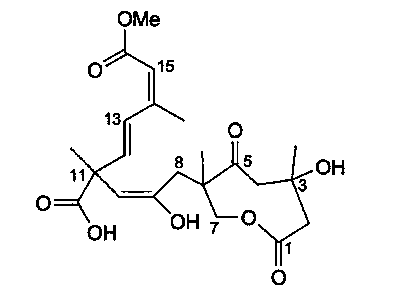
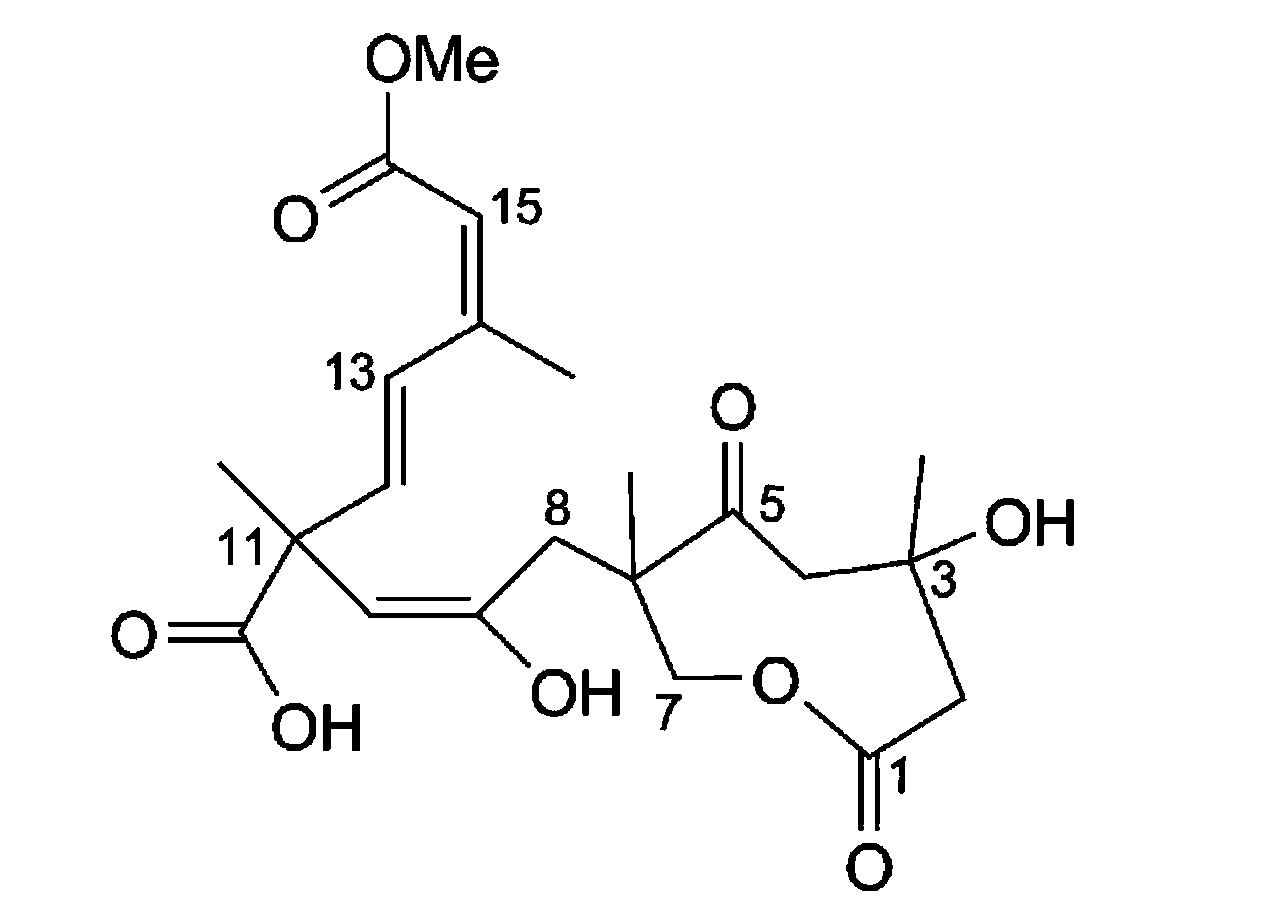
Application of Sarcaboside B in medicine used for treating rheumatoid arthritis
An arthritis and rheumatoid technology, applied in the application field of Sarcaboside B in the treatment of rheumatoid arthritis drugs, can solve the problems of poor action selectivity, ulcers, damage to the gastrointestinal mucosa, etc., to achieve outstanding substantive characteristics and inhibitory activity strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 1: the preparation of compound Sarcaboside B tablet involved in the present invention:
[0013] Take 20 grams of compound Sarcaboside B, add 180 grams of conventional excipients for tablet preparation, mix well, and make 1000 tablets with a conventional tablet press.
Embodiment 2
[0014] Embodiment 2: the preparation of compound Sarcaboside B capsules involved in the present invention:
[0015] Get 20 grams of compound Sarcaboside B, add conventional auxiliary materials for preparing capsules such as 180 grams of starch, mix well, and pack into capsules to make 1000 tablets.
[0016] The following pharmacodynamic experiments will further illustrate its drug activity.
experiment example 1
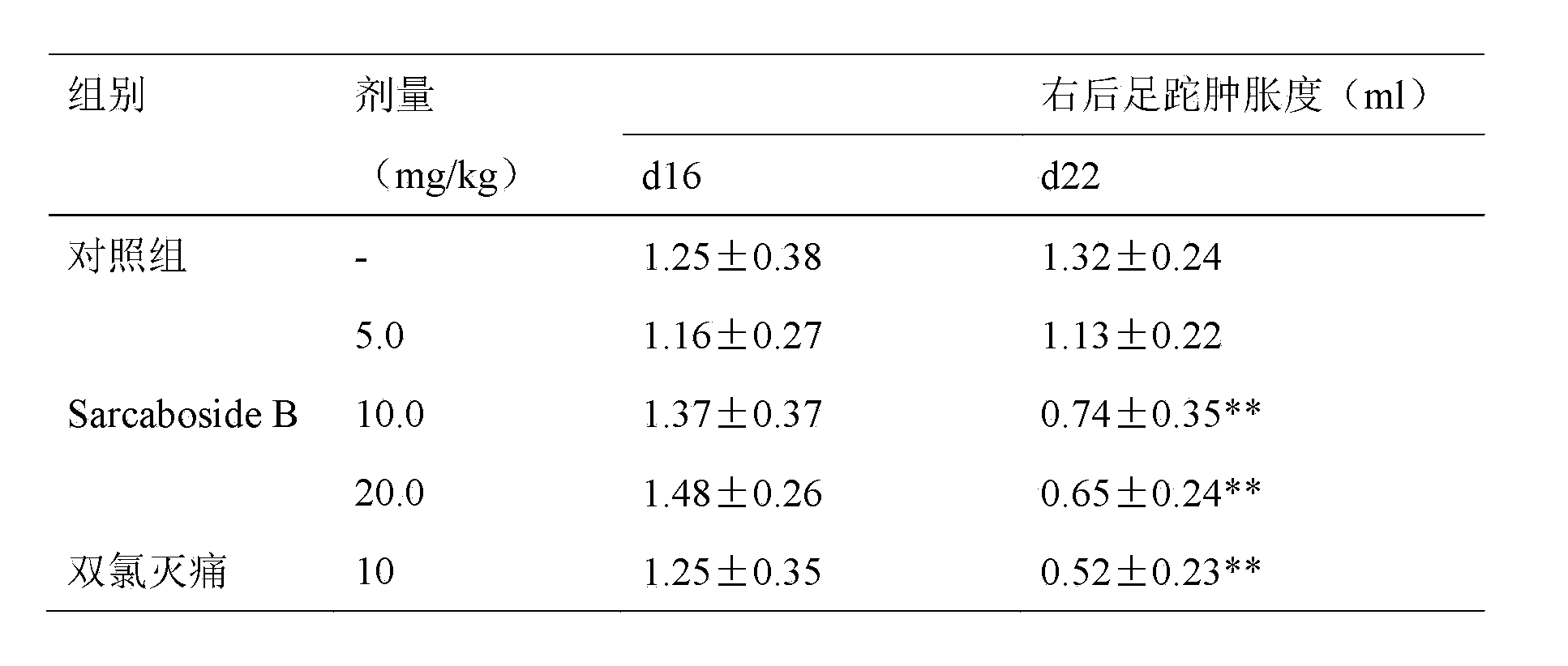
[0017] Experimental example 1, the influence of Sarcaboside B of the present invention on rat adjuvant arthritis
[0018] Grind Mycobacterium casei (Difco) and mix it with liquid paraffin to make 10mg / ml, and make Freund's complete adjuvant after autoclaving. Male SD rats were intradermally injected with 50ul of the above-mentioned adjuvant in the right hind paw, and at the same time, injected 50ul intradermally at the tail about 3cm away from the base of the tail. The difference of , represents the degree of swelling. On the 16th day after the injection of the adjuvant, the swelling of the left hind foot of the rats was obvious. The rats were divided into groups according to the degree of swelling and body weight. The experimental group was orally given Sarcaboside B 5.0, 10.0, 20.0 mg / kg of the present invention, and the positive control group was orally given diclofenac 10 mg / kg, the control group was given equal volume of distilled water, once a day, for 7 consecutive day...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com