Method for producing apolipoprotein in plants
A technology of apolipoproteins and plants, applied in the field of producing apolipoproteins, can solve problems such as inapplicable methods and inability to produce apolipoproteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] Example 1 - Materials and methods
[0144] Cloning and infiltration
[0145] Nucleic acid constructs comprising nucleotide sequences encoding gamma-zein wild-type gene, fragments and variants thereof were linked to synthetic sequences encoding apolipoprotein A1 Milano, respectively. In the case of using a fragment or variant of γ-zein, the nucleic acid construct further comprises a nucleotide sequence encoding the signal peptide of the native γ-zein at the 5' end, if it is present in the fragment or variant words that exist in the body. For some experiments, a synthetic nucleic acid sequence encoding a linker comprising a protease cleavage site between the gamma-zein coding sequence and the apolipoprotein A1 Milano coding sequence was also included in the construct. The coding sequence of apolipoprotein Al Milano has been optimized for expression in plants. The nucleic acid construct is cloned into a vector at a site where expression of the nucleic acid construct in ...
Embodiment 2
[0160] Example 2 - Expression Level of Apolipoprotein A1Milano-γ-Zein Fusion Protein
[0161] A gamma-zein-enterokinase-apolipoprotein A1 Milano fusion protein construct (gamma-zein-ApoA1 ) was prepared as described above and transformed into tobacco plants using the Agrobacterium agroinfiltration method. Total protein was extracted and quantified by Western blotting using γ-zein specific antibodies. A control experiment was also carried out using apolipoprotein A1 Milano (ApoA1 ) expressed in the absence of γ-zein under the same conditions. Expression levels from the mean of 3 Agroinfiltration events were as follows:
[0162] For γ-zein-apolipoprotein A1 Milano, the expression level was between about 3 and 6 g γ-zein-apolipoprotein A1 Milano / kg fresh weight.
[0163] For the gamma zein-free apolipoprotein Al Milano, the expression level was between about 2 and 4 g apolipoprotein / kg fresh weight.
[0164] Based on these average results, it was concluded that the expression ...
Embodiment 3
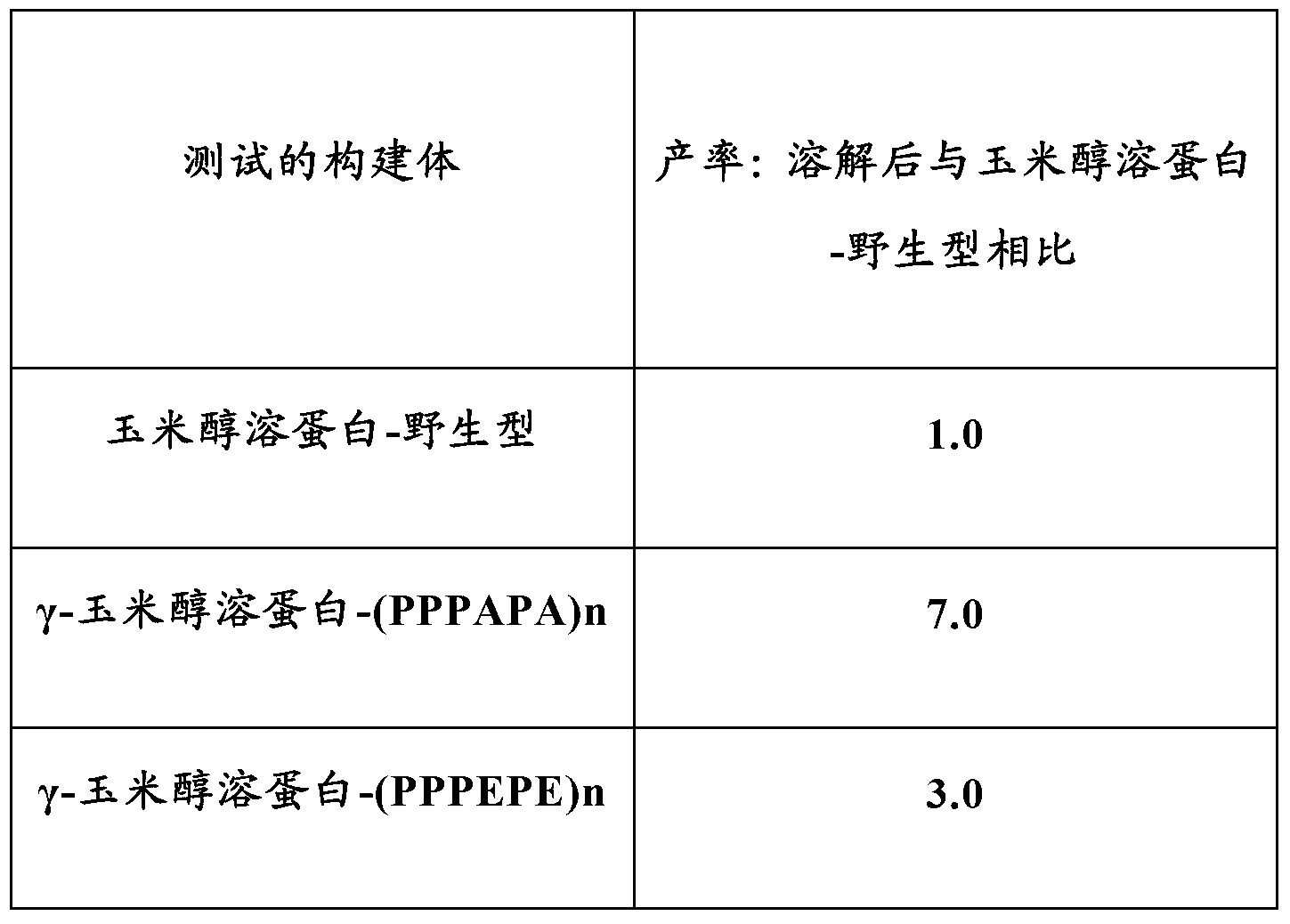
[0165] Example 3 - Analysis of different non-naturally occurring repeat sequence motifs in gamma zein
[0166] A gamma-zein-apolipoprotein A1 Milano fusion construct was prepared using a different non-naturally occurring repeat sequence motif. The following constructs were used: γ-zein peptide only (zein-wild type); γ-zein-(PPPVAL)n; γ-zein-(PPPVEL)n; γ-maize prolamin-(PPPAPA)n; and gamma-zein-(PPPEPE)n.
[0167] The constructs were transformed separately into different tobacco plants using the Agrobacterium infiltration method. Total protein was extracted and quantified by Western blotting using γ-zein specific antibodies. Expression levels from the average of 4 Agroinfiltration events were as follows:
[0168] Constructs tested
The expression level
Zein-wild type
1.0
γ-Zein-(PPPVAL)n
1.5
γ-Zein-(PPPVEL)n
0.85
γ-Zein-(PPPAPA)n
1.81
γ-Zein-(PPPEPE)n
1.27
[0169] Results are expressed as relative q...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com